Label: CLINDAMYCIN PHOSPHATE gel
- NDC Code(s): 45802-900-94, 45802-900-96
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor External Use
-
DESCRIPTION
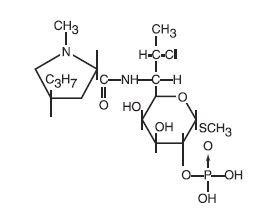
Clindamycin Phosphate Gel USP, 1% contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per gram. Clindamycin phosphate is a water soluble ester of the ...
-
CLINICAL PHARMACOLOGY Mechanism of Action - The mechanism of action of clindamycin in treating acne vulgaris is unknown. Pharmacokinetics - Following multiple topical applications of clindamycin phosphate at a ...
-
INDICATIONS AND USAGE Clindamycin Phosphate Gel is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether ...
-
CONTRAINDICATIONS Clindamycin Phosphate Gel is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative ...
-
WARNINGS Orally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of ...
-
PRECAUTIONS General - Clindamycin phosphate should be prescribed with caution in atopic individuals. Drug Interactions - Clindamycin has been shown to have neuromuscular blocking properties that may ...
-
ADVERSE REACTIONS In 18 clinical studies of various formulations of clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent ...
-
OVERDOSAGE Topically applied clindamycin phosphate can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
-
DOSAGE AND ADMINISTRATION Apply a thin film of Clindamycin Phosphate Gel twice daily to affected area.
-
HOW SUPPLIED Clindamycin Phosphate Gel USP, 1% containing clindamycin phosphate equivalent to 10 mg clindamycin per gram is available in the following sizes: 30 gram tube—NDC 45802-900-94 - 60 gram tube—NDC ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Rx Only - Clindamycin Phosphate Gel USP, 1% For Topical Use Only - 30 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug ...
-
INGREDIENTS AND APPEARANCEProduct Information