Label: CALCIPOTRIENE AND BETAMETHASONE DIPROPIONATE- calcipotriene, betamethasone dipropionate ointment
- NDC Code(s): 45802-816-01, 45802-816-96
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CALCIPOTRIENE and BETAMETHASONE DIPROPIONATE OINTMENT safely and effectively. See Full Prescribing Information for CALCIPOTRIENE and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECalcipotriene and Betamethasone Dipropionate Ointment is indicated for the topical treatment of plaque psoriasis in patients 12 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONApply an adequate layer of Calcipotriene and Betamethasone Dipropionate Ointment to the affected area(s) once daily for up to 4 weeks. Calcipotriene and Betamethasone Dipropionate Ointment should ...
-
3 DOSAGE FORMS AND STRENGTHSOintment, 0.005%/0.064% Each gram of Calcipotriene and Betamethasone Dipropionate Ointment, 0.005%/0.064% contains 50.00 mcg of calcipotriene and 0.643 mg of betamethasone dipropionate (equivalent ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypercalcemia and Hypercalciuria - Hypercalcemia and hypercalciuria have been observed with use of calcipotriene and betamethasone dipropionate ointment. If hypercalcemia or hypercalciuria ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Calcipotriene and Betamethasone Dipropionate Ointment contains calcipotriene and bethamethasone dipropionate. The limited data with Calcipotriene and Betamethasone ...
-
10 OVERDOSAGETopically applied Calcipotriene and Betamethasone Dipropionate Ointment can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1, 5.2)].
-
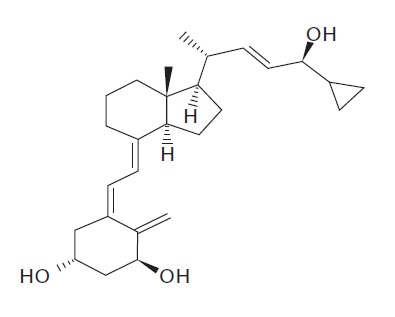
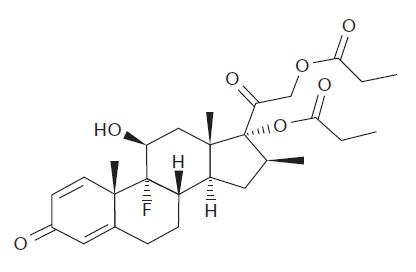
11 DESCRIPTIONCalcipotriene and Betamethasone Dipropionate Ointment, 0.005%/0.064% contains calcipotriene and betamethasone dipropionate. It is intended for topical use only. Calcipotriene is a synthetic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Calcipotriene and Betamethasone Dipropionate Ointment combines the pharmacological effects of calcipotriene as a synthetic vitamin D3 analogue and betamethasone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10 and 30 mcg/kg/day (9, 30 and 90 mcg/m2/day ...
-
14 CLINICAL STUDIESClinical Trials Conducted in Subjects 18 years and older with Plaque Psoriasis - In an international, multi-center, double-blind, vehicle- and active-controlled, parallel-group trial, 1603 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Calcipotriene and Betamethasone Dipropionate Ointment, 0.005%/0.064% is off-white to yellow in color, available in collapsible tubes of: 60 gram (NDC 45802-816-96) 100 gram ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information) Inform patients of the following: • Instruct adult patients (18 years and older) not to use more than 100 g per week. • Instruct pediatric ...
-
PATIENT INFORMATIONCalcipotriene (kal-si-POE-try-een) and Betamethasone (bay-ta-METH-a-sone) Dipropionate Ointment, 0.005%/0.064% Read the Patient Information that comes with Calcipotriene and Betamethasone ...
-
Package/Label Display Panel – 60 g CartonNDC 45802-816-96 - Rx Only - Calcipotriene and Betamethasone Dipropionate Ointment, 0.005%/0.064% For Topical Use Only - NET WT 60 g
-
INGREDIENTS AND APPEARANCEProduct Information