Label: TERCONAZOLE suppository
- NDC Code(s): 45802-717-00, 45802-717-08
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
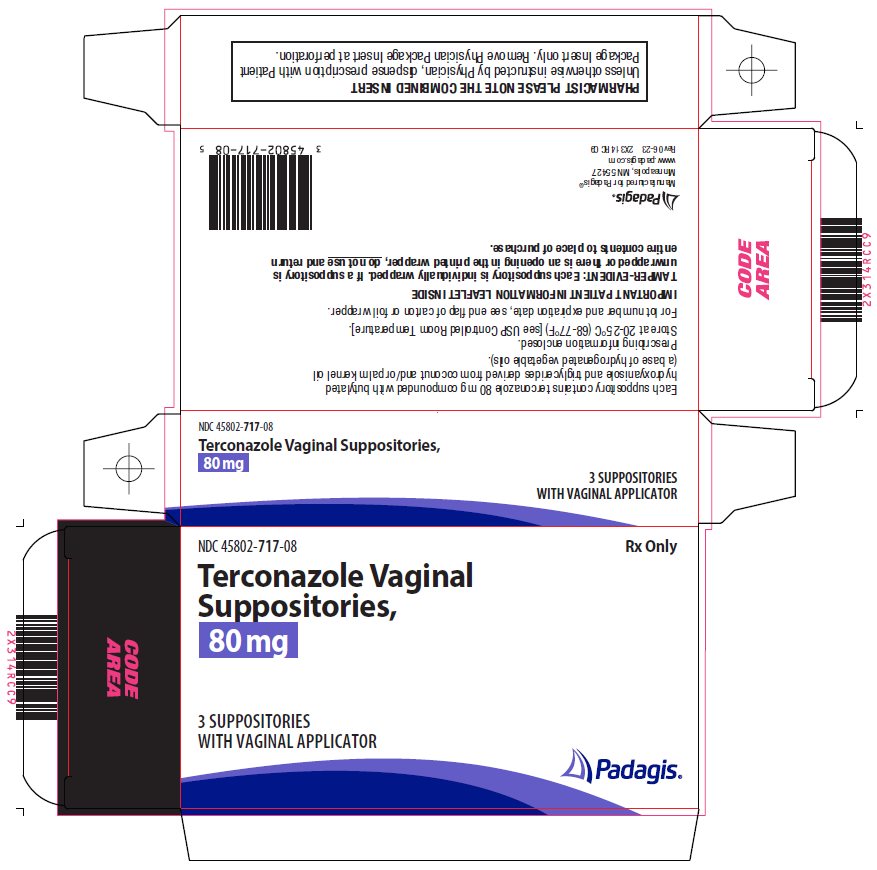
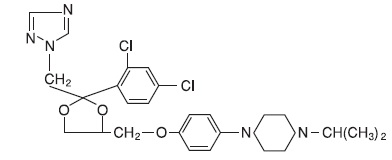
DESCRIPTIONTerconazole Vaginal Suppositories, 80 mg are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole ...
-
CLINICAL PHARMACOLOGYAbsorption - Following a single intravaginal application of a suppository containing 240 mg 14C-terconazole to healthy women, approximately 70% (range: 64-76%) of terconazole remains in the ...
-
INDICATIONS AND USAGETerconazole Vaginal Suppositories, 80 mg are indicated for the local treatment of vulvovaginal candidiasis (moniliasis). As this product is effective only for vulvovaginitis caused by the genus ...
-
CONTRAINDICATIONSPatients known to be hypersensitive to terconazole or to any of the components of the suppositories.
-
WARNINGSAnaphylaxis and toxic epidermal necrolysis have been reported during terconazole therapy. Terconazole Vaginal Suppositories, 80 mg therapy should be discontinued if anaphylaxis or toxic epidermal ...
-
PRECAUTIONSGeneral - For vulvovaginal use only. Terconazole Vaginal Suppositories, 80 mg is not for ophthalmic or oral use. Discontinue use and do not retreat with terconazole if sensitization ...
-
ADVERSE REACTIONSAdverse Reactions from Clinical Trials - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
OVERDOSAGEIn the rat, the oral LD 50 values were found to be 1741 and 849 mg/kg for the male and female, respectively. The oral LD 50 values for the male and female dog were ≅1280 and ≥640 mg/kg ...
-
DOSAGE AND ADMINISTRATIONOne Terconazole Vaginal Suppository, 80 mg should be administered intravaginally once daily at bedtime for three consecutive days. Before prescribing another course of therapy, the diagnosis ...
-
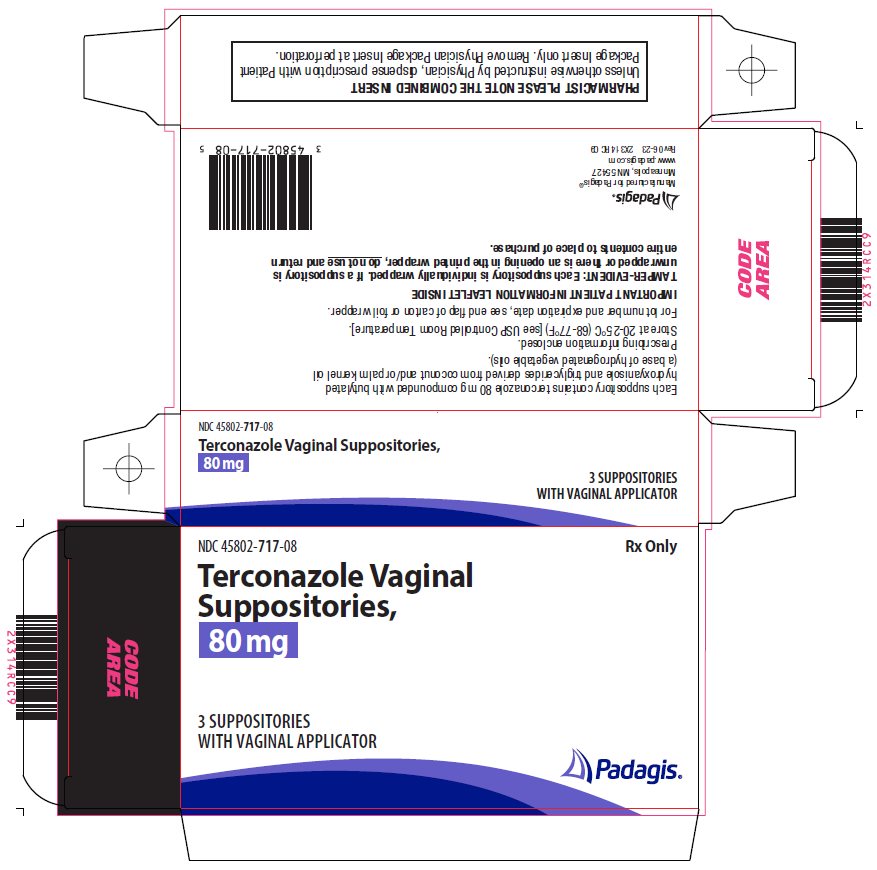
HOW SUPPLIEDTerconazole Vaginal Suppositories, 80 mg are available as follows: Carton containing 3 suppositories (NDC 45802-717-08)
-
STORAGEStore at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
-
SPL UNCLASSIFIED SECTIONManufactured for Padagis® Minneapolis, MN 55427 - www.padagis.com - Rev 09-24 - 2X300 RC J13
-
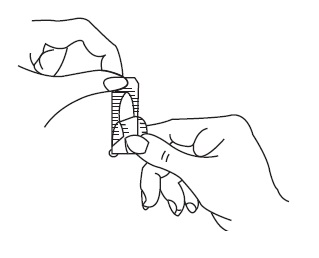
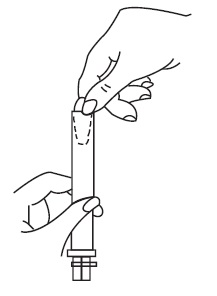
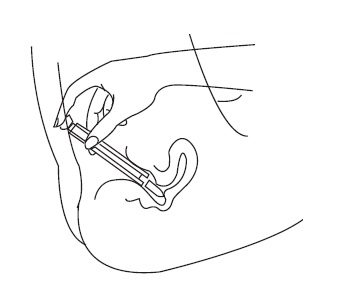
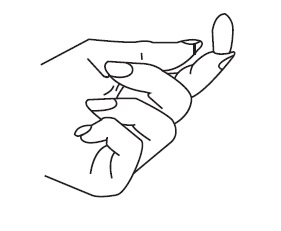
Patient InstructionsTerconazole Vaginal Suppositories, 80 mg - Three oval suppositories, for use inside the vagina only. Designed to be inserted into the vagina. HOW TO USE: Place one suppository into the vagina ...
-
Package/Label Display PanelRx Only - Terconazole Vaginal Suppositories, 80 mg - 3 SUPPOSITORIES with vaginal applicator
-
INGREDIENTS AND APPEARANCEProduct Information