Label: CLINDAMYCIN PHOSPHATE aerosol, foam
- NDC Code(s): 45802-660-32, 45802-660-33
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLINDAMYCIN PHOSPHATE FOAM safely and effectively. See full prescribing information for CLINDAMYCIN PHOSPHATE FOAM. CLINDAMYCIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClindamycin phosphate foam is indicated for topical application in the treatment of acne vulgaris in patients 12 years and older.
-
2 DOSAGE AND ADMINISTRATIONClindamycin phosphate foam is for topical use only, and not for oral, ophthalmic or intravaginal use. Apply clindamycin phosphate foam once daily to affected areas after the skin is washed with ...
-
3 DOSAGE FORMS AND STRENGTHSClindamycin phosphate foam is a white to off-white thermolabile foam. Clindamycin phosphate foam, 1% contains 10 mg of clindamycin as clindamycin phosphate, USP per gram.
-
4 CONTRAINDICATIONSClindamycin phosphate foam is contraindicated in individuals with a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis (including pseudomembranous ...
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of this product. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Erythromycin - Clindamycin phosphate foam should not be used in combination with topical or oral erythromycin-containing products due to possible antagonism to its clindamycin component. In ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on clindamycin phosphate foam use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Animal ...
-
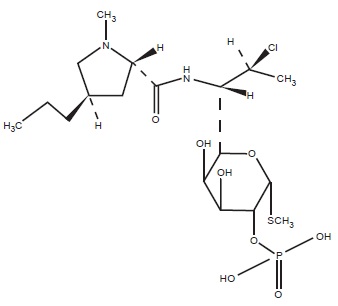
11 DESCRIPTIONClindamycin phosphate foam contains clindamycin (1%) as clindamycin phosphate. Clindamycin phosphate is a water-soluble ester of the semi-synthetic antibiotic produced by a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mechanism of action of clindamycin in acne vulgaris is unknown [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Pharmacodynamics of clindamycin phosphate foam is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The carcinogenicity of a 1.2% clindamycin phosphate gel similar to clindamycin phosphate foam was evaluated by daily topical ...
-
14 CLINICAL STUDIESIn one multicenter, randomized, double-blind, vehicle-controlled clinical trial, subjects with mild to moderate acne vulgaris used clindamycin phosphate foam or the vehicle foam once daily for ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Clindamycin Phosphate Foam, 1% contains 10 mg of clindamycin as clindamycin phosphate, USP per gram. The white to off-white thermolabile foam is available as follows: • 50 ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved patient labeling (Patient Information). 17.1 Instructions for Use - • Patients should be advised to wash their skin with mild soap and allow it to dry before applying ...
-
Patient InformationPatient Information - Clindamycin Phosphate (klin-da-MYE-sin fos-fate) Foam, 1% Important Information: Clindamycin phosphate foam is for use on the skin only. Do not use clindamycin phosphate ...
-
Instructions for Use Instructions for Use - Clindamycin Phosphate (klin-da-MYE-sin fos-fate) Foam, 1% Important Information: Clindamycin phosphate foam is for use on the skin only. Do not use clindamycin phosphate ...
-
Principal Display Panel - 50 g CartonNDC 45802-660-32 - Rx Only - Clindamycin Phosphate Foam, 1% 50g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label ...
-
INGREDIENTS AND APPEARANCEProduct Information