Label: CLINDAMYCIN AND BENZOYL PEROXIDE kit
- NDC Code(s): 45802-510-03, 45802-738-03, 45802-998-03
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTopical Gel: clindamycin (1%) as clindamycin phosphate, benzoyl peroxide (5%) For Dermatological Use Only - Not for Ophthalmic Use - *Reconstitute Before Dispensing*
-
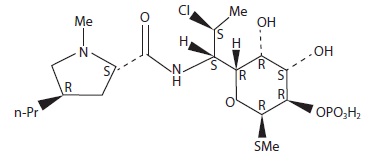
DESCRIPTIONClindamycin and benzoyl peroxide topical gel, 1%/5% contains clindamycin phosphate, (7(S)-chloro-7-deoxylincomycin-2-phosphate). Clindamycin phosphate is a water soluble ester of the ...
-
CLINICAL PHARMACOLOGYAn in vitro percutaneous penetration study comparing clindamycin and benzoyl peroxide topical gel, 1%/5% and topical 1% clindamycin gel alone, demonstrated there was no statistical difference in ...
-
CLINICAL STUDIESIn two adequate and well controlled clinical studies of 758 patients, 214 used clindamycin and benzoyl peroxide topical gel, 1%/5%, 210 used benzoyl peroxide, 168 used clindamycin, and 166 used ...
-
INDICATIONS AND USAGEClindamycin and benzoyl peroxide topical gel, 1%/5% is indicated for the topical treatment of acne vulgaris.
-
CONTRAINDICATIONSClindamycin and benzoyl peroxide topical gel, 1%/5% is contraindicated in those individuals who have shown hypersensitivity to any of its components or to lincomycin. It is also contraindicated ...
-
WARNINGSORALLY AND PARENTERALLY ADMINISTERED CLINDAMYCIN HAS BEEN ASSOCIATED WITH SEVERE COLITIS WHICH MAY RESULT IN PATIENT DEATH. USE OF THE TOPICAL FORMULATION OF CLINDAMYCIN RESULTS IN ABSORPTION OF ...
-
PRECAUTIONSGeneral: For dermatological use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially ...
-
ADVERSE REACTIONSDuring clinical trials, the most frequently reported adverse event in the clindamycin and benzoyl peroxide topical gel, 1%/5% treatment group was dry skin (12%). The Table below lists local ...
-
DOSAGE AND ADMINISTRATIONClindamycin and benzoyl peroxide topical gel, 1%/5% should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is gently washed, rinsed ...
-
HOW SUPPLIED AND COMPOUNDING INSTRUCTIONSSize (Net Weight) NDC 45802 - Benzoyl Peroxide Gel - Active Clindamycin Powder (In plastic vial) Purified Water To Be Added to each vial - 25 grams - 509-01 - 19.7g - 0.3g - 5 ...
-
STORAGE AND HANDLINGStore at room temperature up to 25ºC (77ºF) {See USP}. Do not freeze. Keep tightly closed. Keep out of the reach of children.
-
SPL UNCLASSIFIED SECTIONRx Only - Manufactured By Padagis - Yeruham, Israel - Distributed By PadagisTM - Allegan, MI 49010 - www.padagis.com - Rev 03-22 - 8R500 RC J6
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 35 g CartonRx Only - Clindamycin and Benzoyl Peroxide Topical Gel, 1%/5% For Topical Use Only - Reconstitute with clindamycin phosphate vial - One 35g Pump (after reconstitution) The following image is a ...
-
INGREDIENTS AND APPEARANCEProduct Information