Label: TAZAROTENE gel
- NDC Code(s): 45802-436-01, 45802-436-94, 45802-442-01, 45802-442-94
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAZAROTENE GEL safely and effectively. See full prescribing information for TAZAROTENE GEL. TAZAROTENE gel, 0.05% and 0.1%, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Plaque Psoriasis - Tazarotene gel, 0.05% and 0.1% are indicated for the topical treatment of patients with plaque psoriasis of up to 20% body surface area involvement. 1.2 Acne Vulgaris ...
-
2 DOSAGE AND ADMINISTRATION Tazarotene gel is for topical use only. Tazarotene gel is not for ophthalmic, oral, or intravaginal use. Avoid accidental transfer of tazarotene gel into eyes, mouth, or other mucous membranes. If ...
-
3 DOSAGE FORMS AND STRENGTHS Gel, 0.05% and 0.1%, in 30 g and 100 g tubes. Each gram of Tazarotene Gel, 0.05% and 0.1% contains 0.5 mg and 1 mg of tazarotene, respectively in a translucent, aqueous gel.
-
4 CONTRAINDICATIONS Tazarotene gel is contraindicated in: • Pregnancy. Retinoids may cause fetal harm when administered to a pregnant female [see Warnings and Precautions (5.1), Use in Specific Populations (8.1 ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Embryofetal Toxicity - Based on data from animal reproduction studies, retinoid pharmacology and the potential for systemic absorption, tazarotene gel may cause fetal harm when administered ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in more detail in other sections of the labeling: • Embryofetal toxicity [see Warnings and Precautions (5.1)] • Photosensitivity and Risk ...
-
7 DRUG INTERACTIONS No formal drug-drug interaction studies were conducted with tazarotene gel. In a trial of 27 healthy female subjects between the ages of 20–55 years receiving a combination oral contraceptive ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, tazarotene gel may cause fetal harm when ...
-
10 OVERDOSAGE Excessive topical use of tazarotene gel, 0.05% and 0.1% may lead to marked redness, peeling, or discomfort [see Warnings and Precautions (5.2)]. Tazarotene gel, 0.05% and 0.1% are not for oral ...
-
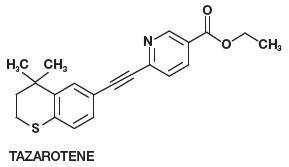
11 DESCRIPTION Tazarotene Gel, 0.05% and 0.1% is for topical use and contains the active ingredient, tazarotene. Each gram of Tazarotene Gel, 0.05% and 0.1% contains 0.5 and 1 mg of tazarotene, respectively in a ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tazarotene is a retinoid prodrug which is converted to its active form, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of ...
-
14 CLINICAL STUDIES Psoriasis: In two large vehicle-controlled clinical trials, tazarotene gel, 0.05% and 0.1% applied once daily for 12 weeks was significantly more effective than vehicle in reducing the severity ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Tazarotene gel is a translucent, aqueous gel, available in concentrations of 0.05% and 0.1%. It is available in a collapsible aluminum tube with a tamper-evident aluminum membrane over the opening ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Embryofetal Toxicity - Inform females of reproductive potential of the potential risk to a fetus. Advise these ...
-
PATIENT INFORMATION PATIENT INFORMATION - Tazarotene (TAZ-AR-oh-teen) Gel, 0.05% and 0.1% Important information: tazarotene gel is for use on skin only. Do not use tazarotene gel in your eyes, mouth, or ...
-
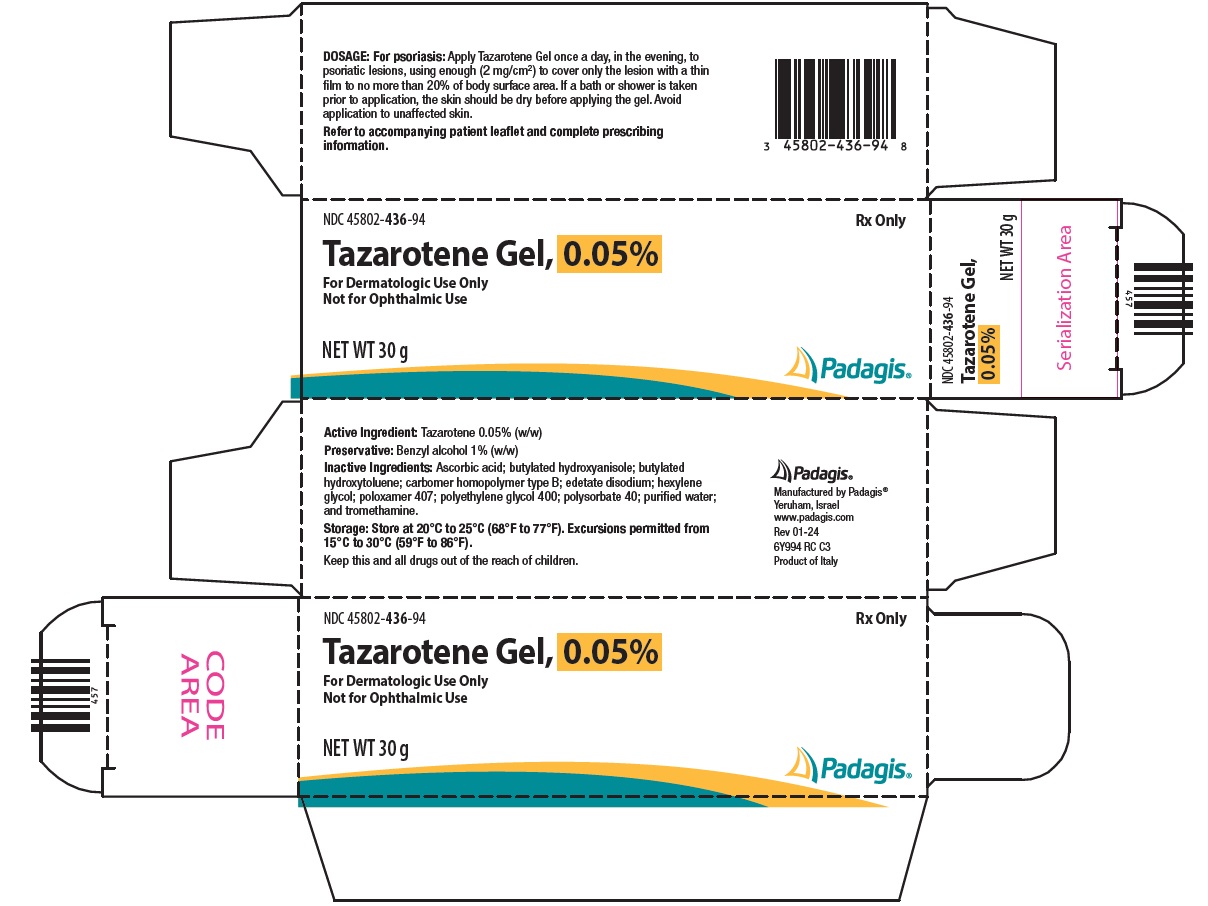
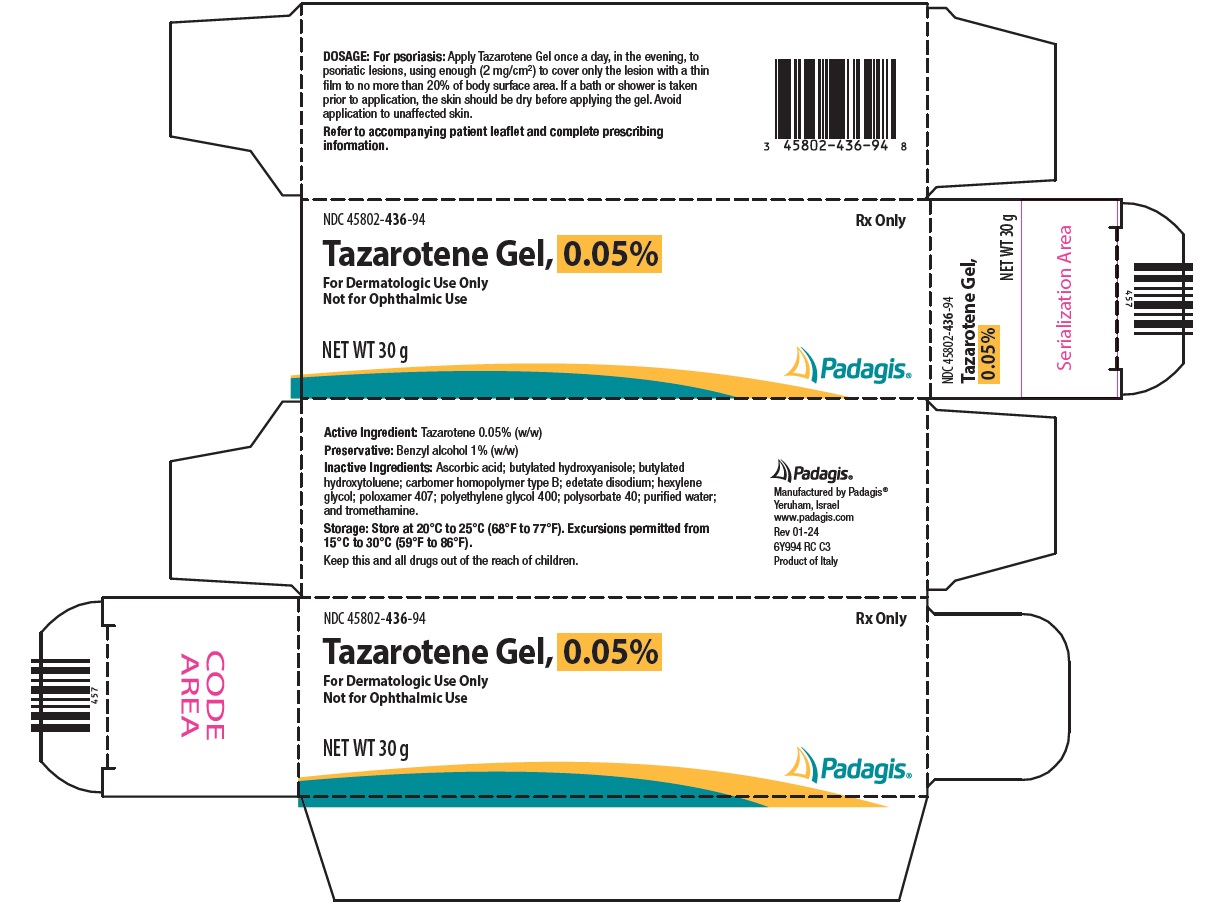
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 45802-436-94 - Rx Only - Tazarotene Gel, 0.05% For Dermatologic Use Only - Not for Ophthalmic Use - NET WT 30 g - The following image is a placeholder representing the product identifier that is ...
-
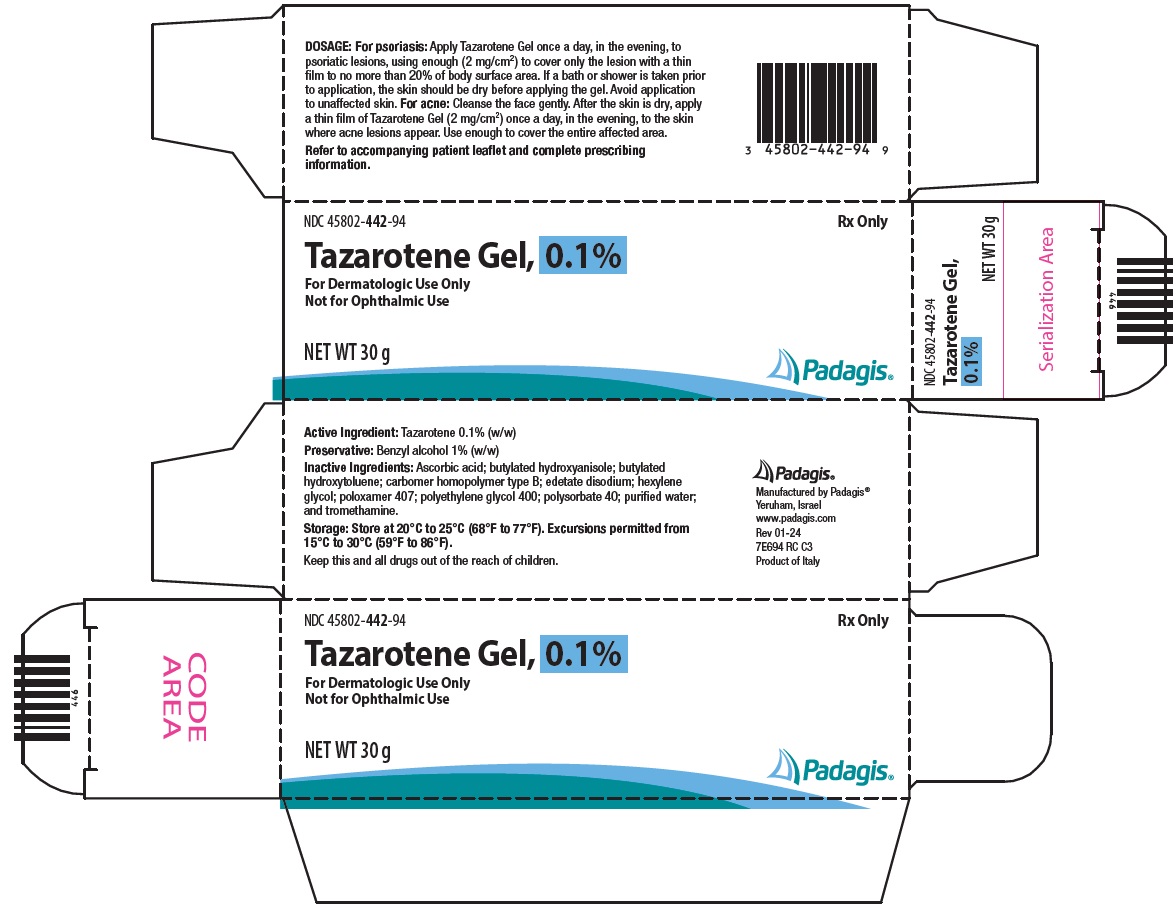
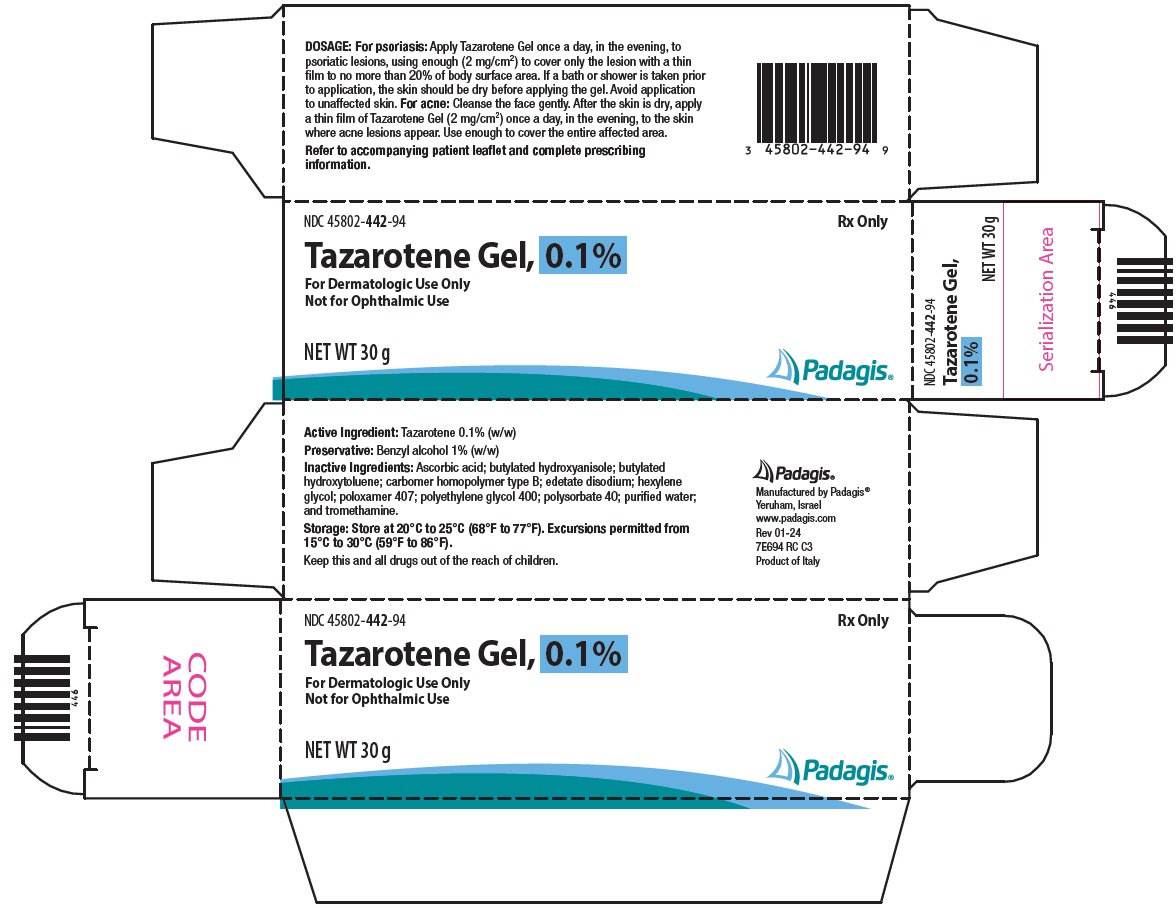
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 45802-442-94 - Rx Only - Tazarotene Gel, 0.1% For Dermatologic Use Only - Not for Ophthalmic Use - NET WT 30 g - The following image is a placeholder representing the product identifier that is either ...
-
INGREDIENTS AND APPEARANCEProduct Information