Label: CICLOPIROX shampoo
- NDC Code(s): 45802-401-09
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CICLOPIROX SHAMPOO safely and effectively. See full prescribing information for CICLOPIROX SHAMPOO.
CICLOPIROX shampoo, for topical use
Initial U.S. Approval: 1982INDICATIONS AND USAGE
- •
- Ciclopirox Shampoo, 1% is an antifungal indicated for the topical treatment of seborrheic dermatitis of the scalp in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- •
- Shampoo, 1% (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- •
- The most frequently reported adverse reactions are pruritus, burning, and erythema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Ciclopirox Shampoo, 1% is not for ophthalmic, oral, or intravaginal use.
Wet hair and apply approximately 1 teaspoon (5 mL) of Ciclopirox Shampoo, 1% to the scalp. Up to 2 teaspoons (10 mL) may be used for long hair. Lather and leave on hair and scalp for 3 minutes. A timer may be used. Avoid contact with eyes. Rinse off. Treatment should be repeated twice per week for 4 weeks, with a minimum of 3 days between applications.
If a patient with seborrheic dermatitis shows no clinical improvement after 4 weeks of treatment with Ciclopirox Shampoo, 1%, the diagnosis should be reviewed.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
If a reaction suggesting sensitivity or irritation occurs with the use of Ciclopirox Shampoo, 1%, treatment should be discontinued and appropriate therapy instituted.
Contact of Ciclopirox Shampoo, 1% with the eyes should be avoided. If contact occurs, rinse thoroughly with water.
In patients with lighter hair color, hair discoloration has been reported.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In 626 subjects treated with ciclopirox shampoo, 1% twice weekly in the two pivotal clinical trials, the most frequent adverse events were increased itching in 1% of subjects, and application site reactions, such as burning, erythema, and itching, also in 1% of subjects.
6.2 Post Marketing Experience
The following adverse reactions have been identified during post approval use of Ciclopirox Shampoo, 1%: hair discoloration and abnormal hair texture, alopecia, irritation and rash. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category B
There are no adequate or well-controlled studies in pregnant women. Therefore, Ciclopirox Shampoo, 1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral embryofetal developmental studies were conducted in mice, rats, rabbits and monkeys. Ciclopirox or ciclopirox olamine was orally administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 77, 125, 80 and 38.5 mg/kg/day ciclopirox in mice, rats, rabbits and monkeys, respectively (approximately 13, 42, 54 and 26 times the maximum recommended human dose based on body surface area comparisons, respectively).
Dermal embryofetal developmental studies were conducted in rats and rabbits with ciclopirox olamine dissolved in PEG 400. Ciclopirox olamine was topically administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 92 mg/kg/day and 77 mg/kg/day ciclopirox in rats and rabbits, respectively (approximately 31 and 54 times the maximum recommended human dose based on body surface area comparisons, respectively).
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Ciclopirox Shampoo, 1% is administered to a nursing woman.
8.5 Geriatric Use
In clinical trials, the safety and tolerability of ciclopirox shampoo, 1% in the population 65 years and older was comparable to that of younger subjects. Results of the efficacy analysis in those subjects 65 years and older showed effectiveness in 25 of 85 (29%) subjects treated with ciclopirox shampoo, 1%, and in 15 of 61 (25%) subjects treated with the vehicle; due to the small sample size, a statistically significant difference was not demonstrated. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity to adverse effects in some older individuals cannot be ruled out.
-
11 DESCRIPTION
Ciclopirox Shampoo, 1% contains the synthetic antifungal agent, ciclopirox for topical use.
Each gram (equivalent to 0.96 mL) of Ciclopirox Shampoo, 1% contains 10 mg ciclopirox in a shampoo base consisting of disodium laureth sulfosuccinate, laureth-2, purified water, sodium chloride, and sodium laureth sulfate.
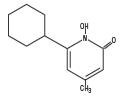
Ciclopirox Shampoo, 1% is a colorless, translucent solution. The chemical name for ciclopirox is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, with the empirical formula C12H17NO2 and a molecular weight of 207.27. The CAS Registry Number is [29342-05-0]. The chemical structure is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ciclopirox is a hydroxypyridone antifungal agent although the relevance of this property for the indication of seborrheic dermatitis is not known. Ciclopirox acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.
12.3 Pharmacokinetics
In a study in patients with seborrheic dermatitis of the scalp, application of 5 mL ciclopirox shampoo 1% twice weekly for 4 weeks, with an exposure time of 3 minutes per application, resulted in detectable serum concentrations of ciclopirox in 6 out of 18 patients. The serum concentrations measured throughout the dosing interval on Days 1 and 29 ranged from 10.3 ng/mL to 13.2 ng/mL. Total urinary excretion of ciclopirox was less than 0.5% of the administered dose.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week dermal carcinogenicity study in mice was conducted with ciclopirox cream applied at doses up to 1.93% (100 mg/kg/day or 300 mg/m2/day). No increase in drug related neoplasms was noted when compared to control.
The following in vitro genotoxicity tests have been conducted with ciclopirox: evaluation of gene mutation in the Ames Salmonella and E. coli assays (negative); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells, with and without metabolic activation (positive); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells in the presence of supplemental Fe3+, with and without metabolic activation (negative); gene mutation assays in the HGPRT-test with V79 Chinese hamster lung fibroblast cells (negative); and a primary DNA damage assay (i.e., unscheduled DNA synthesis assay in A549 human cells) (negative). An in vitro cell transformation assay in BALB/c 3T3 cells was negative for cell transformation. In an in vivo Chinese hamster bone marrow cytogenetic assay, ciclopirox was negative for chromosome aberrations at a dosage of 5000 mg/kg body weight.
A combined oral fertility and embryofetal developmental study was conducted in rats with ciclopirox olamine. No effect on fertility or reproductive performance was noted at the highest dose tested of 3.85 mg/kg/day ciclopirox (approximately 1.3 times the maximum recommended human dose based on body surface area comparisons).
-
14 CLINICAL STUDIES
In two randomized, double-blind clinical trials, subjects 16 years and older with seborrheic dermatitis of the scalp applied ciclopirox shampoo, 1% or its vehicle twice weekly for 4 weeks. Subjects who were immunocompromised, those with psoriasis or atopic dermatitis, women of childbearing potential not using adequate contraception, and pregnant or lactating women were excluded from the clinical trials. An evaluation of the overall status of the seborrheic dermatitis, the presence and severity of erythema or inflammation, and scaling, was made at week 4, using a scale of 0 = none, 1 = slight, 2 = mild, 3 = moderate, 4 = pronounced, and 5 = severe. Effective treatment was defined as achieving a score of 0 (or a score of 1 if the baseline score was ≥ 3) simultaneously for status of the seborrheic dermatitis, erythema or inflammation, and scaling at Week 4. Ciclopirox shampoo was shown to be statistically significantly more effective than vehicle in both trials. Efficacy results for the two trials are presented in Table 1 below.
Table 1. Effective Treatment Rates at Week 4 in Trials 1 and 2
Ciclopirox Shampoo
Vehicle
Study 1
220/380 (58%)
60/192 (31%)
Study 2
65/250 (26%)
32/249 (13%)
Efficacy for African American subjects was not demonstrated, although only 53 African American subjects were enrolled in the two pivotal trials.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
The patient should be instructed to:
- •
- Use Ciclopirox Shampoo, 1% as directed by the physician. Avoid contact with the eyes. If contact occurs, rinse thoroughly with water. Ciclopirox Shampoo, 1% is for external use on the scalp only. Do not swallow.
- •
- Use Ciclopirox Shampoo, 1% for seborrheic dermatitis for the full treatment time even though symptoms may have improved. Notify the physician if there is no improvement after 4 weeks.
- •
- Inform the physician if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, or oozing).
-
Patient Information
Ciclopirox (sigh-kloe-peer-ox) Shampoo, 1%
Important: For use on the scalp only. Do not get Ciclopirox Shampoo, 1% in your eyes, mouth, or vagina.
What is Ciclopirox Shampoo, 1%?
Ciclopirox Shampoo, 1% is a prescription medicine used on the scalp to treat adults with a skin condition called seborrheic dermatitis.
It is not known if Ciclopirox Shampoo, 1% is safe and effective in children under 16 years of age.
What should I tell my doctor before using Ciclopirox Shampoo, 1%?
Before using Ciclopirox Shampoo, 1%, tell your doctor if you:
- •
- have any other medical conditions
- •
- are pregnant or plan to become pregnant. It is not known if Ciclopirox Shampoo, 1% will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if ciclopirox passes into your breast milk.
- •
- are taking prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Ciclopirox Shampoo, 1%?
- •
- Use Ciclopirox Shampoo, 1% exactly as your doctor tells you to use it.
- •
- Wash your hair using Ciclopirox Shampoo, 1% 2 times each week for 4 weeks. There should be at least 3 days between each time you use Ciclopirox Shampoo, 1%.
- •
- Use Ciclopirox Shampoo, 1% for 4 weeks even if your skin condition improves.
- •
- Tell your doctor if your scalp condition is not getting better after you have used Ciclopirox Shampoo, 1% for 4 weeks.
- •
- Do not swallow Ciclopirox Shampoo, 1%.
- •
- Avoid getting Ciclopirox Shampoo, 1% in your eyes. If Ciclopirox Shampoo, 1% gets into your eyes, rinse them well with water.
How should I apply Ciclopirox Shampoo, 1%?
- •
- Wet your hair and apply approximately 1 teaspoon of Ciclopirox Shampoo, 1% to your scalp. You may use up to 2 teaspoons of Ciclopirox Shampoo, 1% if you have long hair. Lather and leave Ciclopirox Shampoo, 1% on your hair and scalp for 3 minutes. You may use a timer.
- •
- After 3 minutes have passed, rinse your hair and scalp.
What are the possible side effects of Ciclopirox Shampoo, 1%?
The most common side effects of Ciclopirox Shampoo, 1% include: itching, burning, and redness of the scalp. Tell your doctor if you get any of these symptoms and they become worse or do not go away, or if you get blistering, swelling, or oozing in your scalp.
These are not all the possible side effects of Ciclopirox Shampoo, 1%. For more information, ask your doctor.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
How should I store Ciclopirox Shampoo, 1%?
- •
- Store Ciclopirox Shampoo, 1% at room temperature, between 20-25°C (68-77°F).
- •
- Safely throw away any unused Ciclopirox Shampoo, 1% after you finish your treatment.
Keep Ciclopirox Shampoo, 1% and all medicines out of the reach of children.
General information about Ciclopirox Shampoo, 1%
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Ciclopirox Shampoo, 1% for a condition for which it was not prescribed. Do not give Ciclopirox Shampoo, 1% to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about Ciclopirox Shampoo, 1% that is written for health professionals.
For more information about Ciclopirox Shampoo, 1% call 1-866-634-9120.
What are the ingredients in Ciclopirox Shampoo, 1%?
Active ingredient: ciclopirox
Inactive ingredients: disodium laureth sulfosuccinate,
laureth-2, purified water, sodium chloride, and sodium laureth sulfate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by Padagis®, Yeruham, Israel
www.padagis.com
Rev 03-23
62X6M RC F4
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
CICLOPIROX
ciclopirox shampooProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CICLOPIROX (UNII: 19W019ZDRJ) (CICLOPIROX - UNII:19W019ZDRJ) CICLOPIROX 1 g in 100 mL Inactive Ingredients Ingredient Name Strength DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) LAURETH-2 (UNII: D4D38LT1L5) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-401-09 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/17/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078594 02/17/2010 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)