Label: CICLOPIROX OLAMINE suspension
- NDC Code(s): 45802-400-46, 45802-400-49
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - For Topical Use Only - Not for use in eyes
-
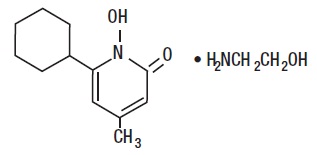
DESCRIPTIONCiclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is for topical use. Each gram of Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) contains 7.70 mg of ciclopirox (as ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Ciclopirox is a hydroxypyridone antifungal agent that acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that ...
-
INDICATIONS AND USAGECiclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris and tinea corporis due to ...
-
CONTRAINDICATIONSCiclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is contraindicated in individuals who have shown hypersensitivity to any of its components.
-
WARNINGSGeneral - Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is not for ophthalmic use. Keep out of reach of children.
-
PRECAUTIONSIf a reaction suggesting sensitivity or chemical irritation should occur with the use of Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion), treatment should be discontinued and ...
-
ADVERSE REACTIONSIn the controlled clinical trial with 89 patients using ciclopirox olamine topical suspension and 89 patients using the vehicle, the incidence of adverse reactions was low. Those considered ...
-
DOSAGE AND ADMINISTRATIONGently massage Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with ...
-
HOW SUPPLIEDCiclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is available as follows: 30 mL bottle (NDC 45802-400-49) 60 mL bottle (NDC 45802-400-46) Bottle space provided to allow for ...
-
STORAGE AND HANDLINGStore at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature]. To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or ...
-
SPL UNCLASSIFIED SECTIONManufactured by Padagis® Yeruham, Israel - www.padagis.com - Rev 01-25 - 98L00 RC PH3
-
Principal Display Panel - CartonNDC 45802-400-49 - Rx Only - Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) For Topical Use Only. Not for use in eyes. Keep Out of Reach of Children. Shake well before use. 30 ...
-
INGREDIENTS AND APPEARANCEProduct Information