Label: CLINDAMYCIN PHOSPHATE solution
- NDC Code(s): 45802-263-37, 45802-263-93
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor External Use - Rx Only
-
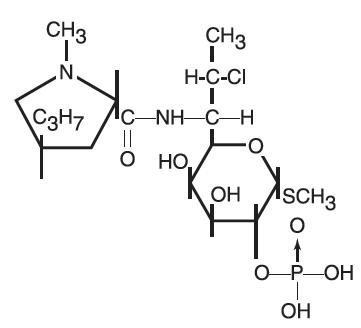
DESCRIPTIONClindamycin Phosphate Topical Solution contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per milliliter. Each Clindamycin Phosphate Topical Solution pledget ...
-
CLINICAL PHARMACOLOGY Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin. Cross resistance has been demonstrated between ...
-
INDICATIONS AND USAGEClindamycin Phosphate Topical Solution USP, 1% is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician ...
-
CONTRAINDICATIONSClindamycin Phosphate Topical Solution USP, 1% is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional ...
-
WARNINGSOrally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of ...
-
PRECAUTIONSGeneral - Clindamycin Phosphate Topical Solution USP, 1% contains an alcohol base which will cause burning and irritation of the eye. In the event of accidental contact with sensitive surfaces ...
-
ADVERSE REACTIONSIn 18 clinical studies of various formulations of topical clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment ...
-
OVERDOSAGETopically applied Clindamycin Phosphate Topical Solution USP, 1% can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
-
DOSAGE AND ADMINISTRATIONDo not use if the seal on jar is broken. Remove pledget from jar just before use. Use a pledget to apply a thin film of Clindamycin Phosphate Topical Solution to the affected area twice daily ...
-
HOW SUPPLIEDClindamycin Phosphate Topical Solution USP, 1% is available as follows: A jar containing 60 single-use pledget applicators (NDC 45802-263-37) A jar containing 69 single-use pledget applicators ...
-
STORAGEStore at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Protect from freezing.
-
SPL UNCLASSIFIED SECTIONManufactured By Padagis - Yeruham, Israel - Distributed By Padagis - Allegan, MI 49010 - www.padagis.com - Rev 11-22 - 74P33 RC F3
-
Package/Label Display Panel Rx Only - NDC 45802-263-37 - Clindamycin Phosphate Topical Solution USP, 1%* (Pledgets) *equivalent to 1% (10 mg/mL) clindamycin - For Topical Use Only - 60 Pledgets - The following image is a ...
-
INGREDIENTS AND APPEARANCEProduct Information