Label: CICLOPIROX OLAMINE cream
- NDC Code(s): 45802-138-11, 45802-138-18, 45802-138-35
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
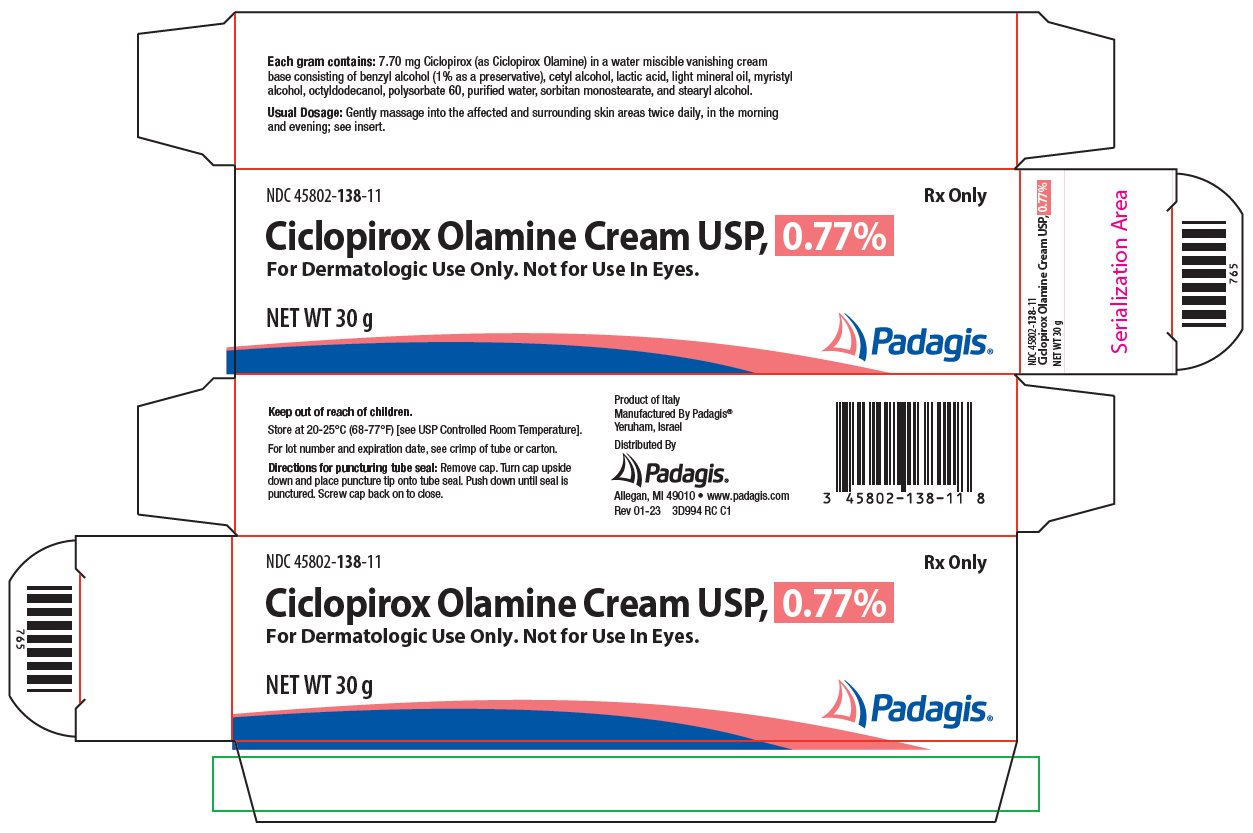
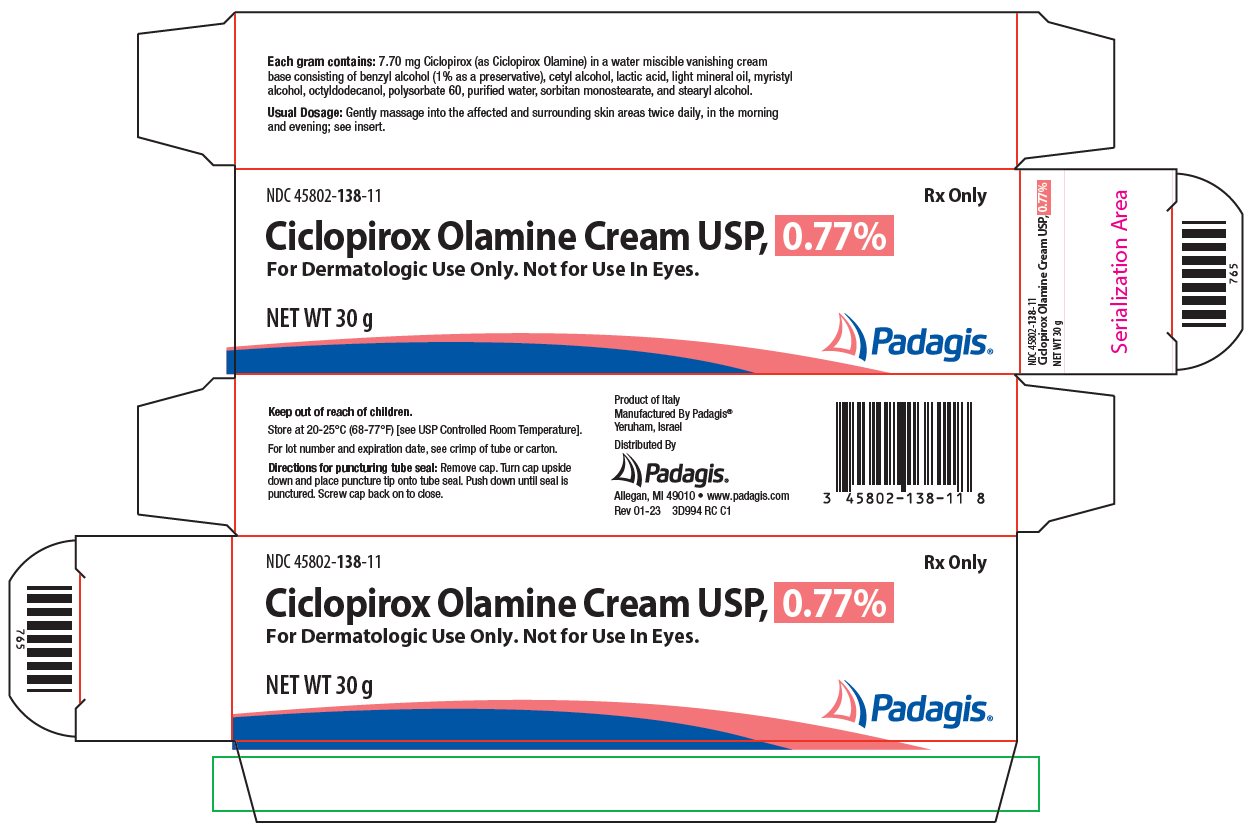
Ciclopirox Olamine Cream USP, 0.77% is for topical use.
Each gram of ciclopirox olamine cream contains 7.70 mg of ciclopirox (as ciclopirox olamine) in a water miscible, vanishing cream base consisting of benzyl alcohol (1% as a preservative), cetyl alcohol, lactic acid, light mineral oil, myristyl alcohol, octyldodecanol, polysorbate 60, purified water, sorbitan monostearate, and stearyl alcohol.
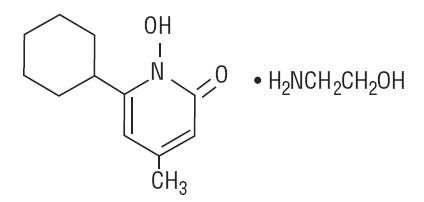
Ciclopirox olamine cream contains a synthetic, broad-spectrum, antifungal agent ciclopirox (as ciclopirox olamine). The chemical name is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, 2-aminoethanol salt.
The CAS Registry Number is 41621-49-2. The chemical structure is:
Ciclopirox olamine cream has a pH of 7.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Ciclopirox is a hydroxypyridone antifungal agent that acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.
Pharmacokinetics
Pharmacokinetic studies in men with tagged ciclopirox solution in polyethylene glycol 400 showed an average of 1.3% absorption of the dose when it was applied topically to 750 cm2 on the back followed by occlusion for 6 hours. The biological half-life was 1.7 hours and excretion occurred via the kidney. Two days after application only 0.01% of the dose applied could be found in the urine. Fecal excretion was negligible.
Penetration studies in human cadaverous skin from the back, with ciclopirox olamine cream with tagged ciclopirox showed the presence of 0.8 to 1.6% of the dose in the stratum corneum 1.5 to 6 hours after application. The levels in the dermis were still 10 to 15 times above the minimum inhibitory concentrations.
Autoradiographic studies with human cadaverous skin showed that ciclopirox penetrates into the hair and through the epidermis and hair follicles into the sebaceous glands and dermis, while a portion of the drug remains in the stratum corneum.
Draize Human Sensitization Assay, 21-Day Cumulative Irritancy study, Phototoxicity study, and Photo-Draize study conducted in a total of 142 healthy male subjects showed no contact sensitization of the delayed hypersensitivity type, no irritation, no phototoxicity, and no photo-contact sensitization due to ciclopirox olamine cream.
-
INDICATIONS AND USAGE
Ciclopirox olamine cream is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis (moniliasis) due to Candida albicans; and tinea (pityriasis) versicolor due to Malassezia furfur.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of ciclopirox olamine cream, treatment should be discontinued and appropriate therapy instituted.
Information for Patients
The patient should be told to:
- 1.
- Use the medication for the full treatment time even though symptoms may have improved and notify the physician if there is no improvement after four weeks.
- 2.
- Inform the physician if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, or oozing) indicative of possible sensitization.
- 3.
- Avoid the use of occlusive wrappings or dressings.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
A 104-week dermal carcinogenicity study in mice was conducted with ciclopirox cream applied at doses up to 1.93% (100 mg/kg/day or 300 mg/m2/day). No increase in drug related neoplasms was noted when compared to control.
The following in vitro genotoxicity tests have been conducted with ciclopirox: evaluation of gene mutation in the Ames Salmonella and E. coli assays (negative); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells, with and without metabolic activation (positive); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells in the presence of supplemental Fe3+, with and without metabolic activation (negative); gene mutation assays in the HGPRT-test with V79 Chinese hamster lung fibroblast cells (negative); and a primary DNA damage assay (i.e., unscheduled DNA synthesis assay in A549 human cells) (negative). An in vitro cell transformation assay in BALB/c 3T3 cells was negative for cell transformation. In an in vivo Chinese hamster bone marrow cytogenetic assay, ciclopirox was negative for chromosome aberrations at a dosage of 5000 mg/kg body weight.
A combined oral fertility and embryofetal developmental study was conducted in rats with ciclopirox olamine. No effect on fertility or reproductive performance was noted at the highest dose tested of 3.85 mg/kg/day ciclopirox (approximately 1.2 times the maximum recommended human dose based on body surface area comparisons).
Pregnancy
Teratogenic Effects: Pregnancy Category B
There are no adequate or well-controlled studies in pregnant women. Therefore, ciclopirox olamine cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral embryofetal developmental studies were conducted in mice, rats, rabbits and monkeys. Ciclopirox or ciclopirox olamine was orally administered during the period of organogenesis. No maternal toxicity, embryotoxicity, or teratogenicity were noted at the highest doses of 77, 125, 80 and 38.5 mg/kg/day ciclopirox in mice, rats, rabbits and monkeys, respectively (approximately 11, 37, 51 and 24 times the maximum recommended human dose based on body surface area comparisons, respectively).
Dermal embryofetal developmental studies were conducted in rats and rabbits with ciclopirox olamine dissolved in PEG 400. Ciclopirox olamine was topically administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 92 mg/kg/day and 77 mg/kg/day ciclopirox in rats and rabbits, respectively (approximately 27 and 49 times the maximum recommended human dose based on body surface area comparisons, respectively).
-
ADVERSE REACTIONS
In all controlled clinical studies with 514 patients using ciclopirox olamine cream and in 296 patients using the vehicle cream, the incidence of adverse reactions was low. This included pruritus at the site of application in one patient and worsening of the clinical signs and symptoms in another patient using ciclopirox olamine cream and burning in one patient and worsening of the clinical signs and symptoms in another patient using the vehicle cream.
-
DOSAGE AND ADMINISTRATION
Gently massage ciclopirox olamine cream into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms usually occurs within the first week of treatment. If a patient shows no clinical improvement after four weeks of treatment with ciclopirox olamine cream the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
- HOW SUPPLIED
-
STORAGE
Store at 20-25ºC (68-77ºF) [see USP Controlled Room Temperature].
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- SPL UNCLASSIFIED SECTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CICLOPIROX OLAMINE
ciclopirox olamine creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CICLOPIROX OLAMINE (UNII: 50MD4SB4AP) (CICLOPIROX - UNII:19W019ZDRJ) CICLOPIROX 7.7 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETYL ALCOHOL (UNII: 936JST6JCN) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) LIGHT MINERAL OIL (UNII: N6K5787QVP) MYRISTYL ALCOHOL (UNII: V42034O9PU) OCTYLDODECANOL (UNII: 461N1O614Y) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-138-35 1 in 1 CARTON 09/05/2006 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:45802-138-11 1 in 1 CARTON 07/19/2006 2 30 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:45802-138-18 1 in 1 CARTON 12/14/2006 3 90 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077364 07/19/2006 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)