Label: MOMETASONE FUROATE ointment
- NDC Code(s): 45802-119-37, 45802-119-42
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MOMETASONE FUROATE OINTMENT safely and effectively. See full prescribing information for MOMETASONE FUROATE OINTMENT. MOMETASONE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMometasone Furoate Ointment, 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 2 years of age or ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin film of Mometasone Furoate Ointment, 0.1% to the affected skin areas once daily. Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks ...
-
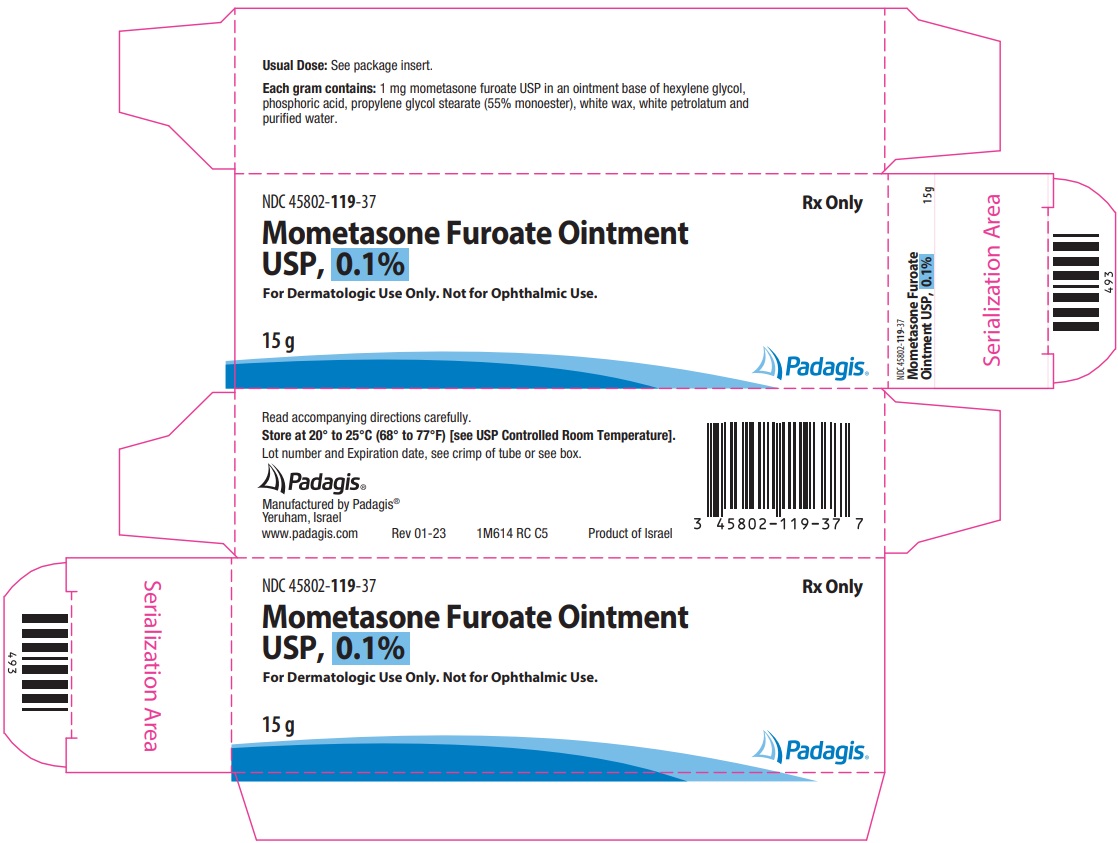
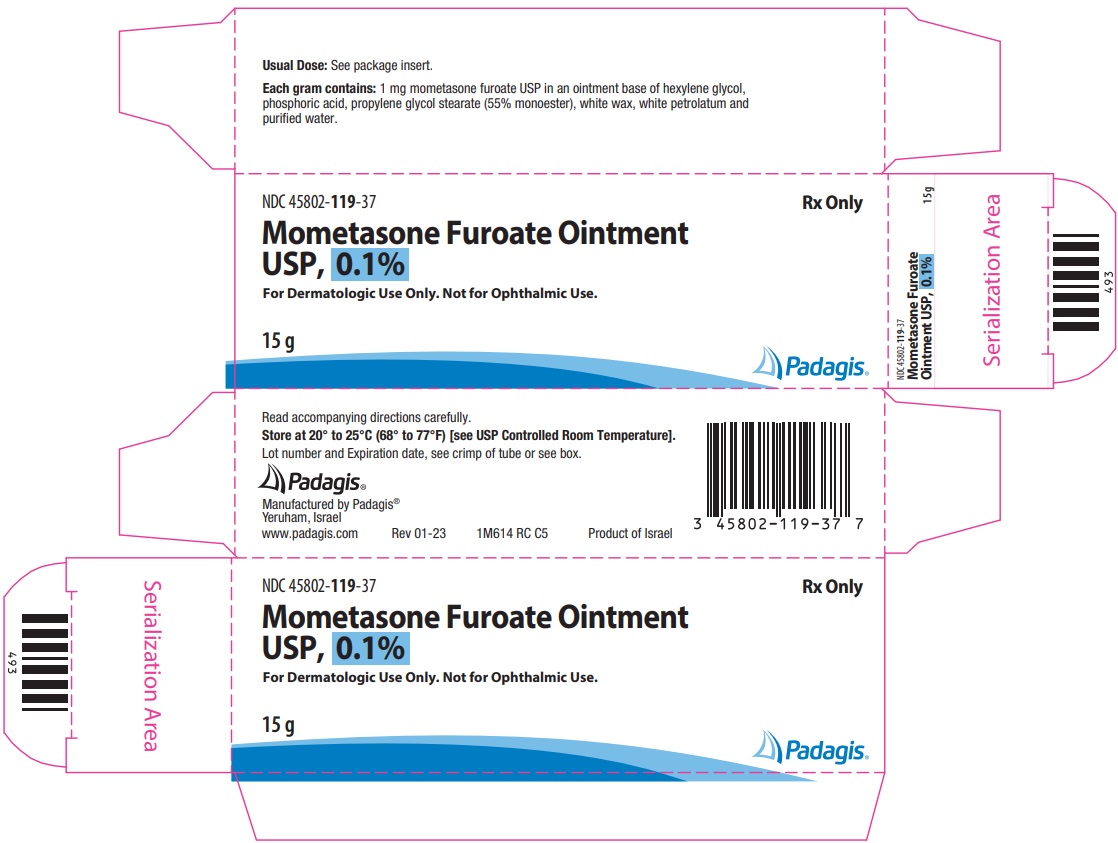
3 DOSAGE FORMS AND STRENGTHSOintment, 0.1%. Each gram of Mometasone Furoate Ointment USP, 0.1% contains 1 mg of mometasone furoate in a white to off-white uniform ointment base.
-
4 CONTRAINDICATIONSMometasone Furoate Ointment, 0.1% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparation.
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on Endocrine System - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 Drug Interactions No drug-drug interaction studies have been conducted with Mometasone Furoate Ointment, 0.1%
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects - Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. Therefore, Mometasone Furoate Ointment, 0.1% should be used ...
-
10 OVERDOSAGETopically applied Mometasone Furoate Ointment, 0.1% can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)].
-
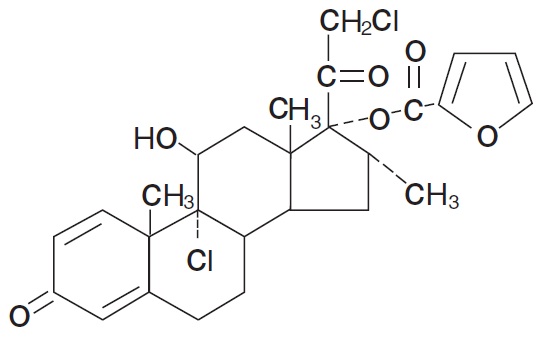
11 DESCRIPTIONMometasone Furoate Ointment USP, 0.1% contains mometasone furoate for topical use. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity. Chemically, mometasone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, mometasone furoate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of mometasone furoate ointment, 0.1%. Long-term ...
-
14 CLINICAL STUDIESThe safety and efficacy of mometasone furoate ointment, 0.1% for the treatment of corticosteroid-responsive dermatoses was demonstrated in two vehicle-controlled trials, one in psoriasis and one ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMometasone Furoate Ointment USP, 0.1% is a white to off-white uniform ointment and supplied in 15-gram (NDC 45802-119-37) and 45-gram (NDC 45802-119-42) tubes; boxes of one. Store at 20° to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients of the following: • Use Mometasone Furoate Ointment, 0.1% as directed by the physician. It is ...
-
Patient Information Mometasone Furoate (moe-MET-a-sone fur-o-ate) Ointment USP, 0.1% Important information: Mometasone Furoate Ointment, 0.1% is for use on skin only. Do not use Mometasone Furoate Ointment, 0.1 ...
-
Package/Label Display PanelNDC 45802-119-37 - Rx Only - Mometasone Furoate Ointment USP, 0.1% For Dermatologic Use Only. Not for Ophthalmic Use. 15 g - The following image is a placeholder representing the product identifier ...
-
INGREDIENTS AND APPEARANCEProduct Information