Label: HYDROCORTISONE cream
HYDROCORTISONE ointment
- NDC Code(s): 45802-004-02, 45802-004-03, 45802-014-02, 45802-014-05
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
DESCRIPTIONEach gram of Hydrocortisone Cream USP, 2.5% contains 25 mg of hydrocortisone in a cream base of cetyl alcohol, methylparaben, propylene glycol, propylparaben, purified water, sodium lauryl ...
-
CLINICAL PHARMACOLOGYTopical corticosteroids share anti-inflammatory, antipruritic, and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various ...
-
INDICATIONS AND USAGETopical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatosis.
-
CONTRAINDICATIONSTopical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and ...
-
Information for the Patient -Patients using topical corticosteroids should receive the following information and instructions: 1. This medication is to be used as directed by the physician. It is for external use only. Avoid ...
-
ADVERSE REACTIONSThe following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONTopical corticosteroids are generally applied to the affected area as a thin film from two to four times daily depending on the severity of the condition. Occlusive dressings may be used for the ...
-
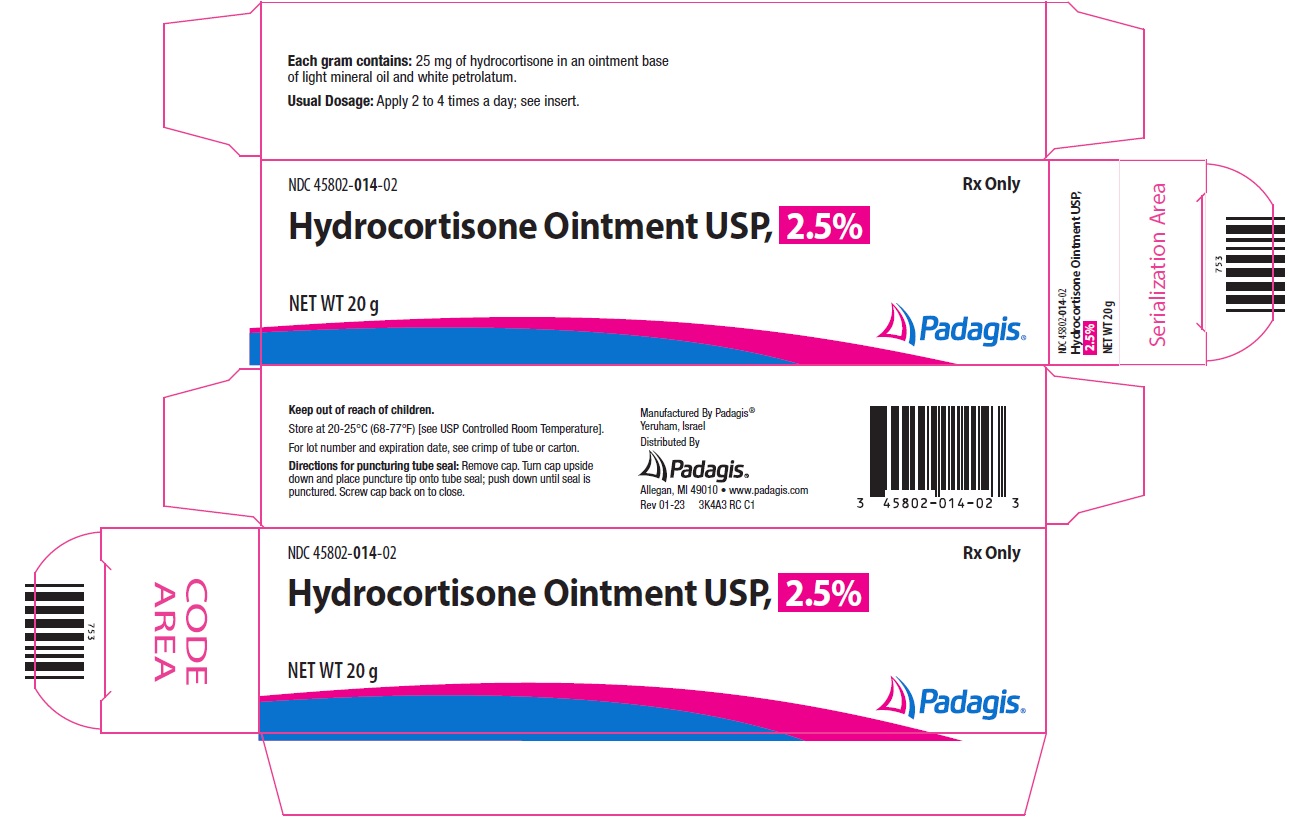
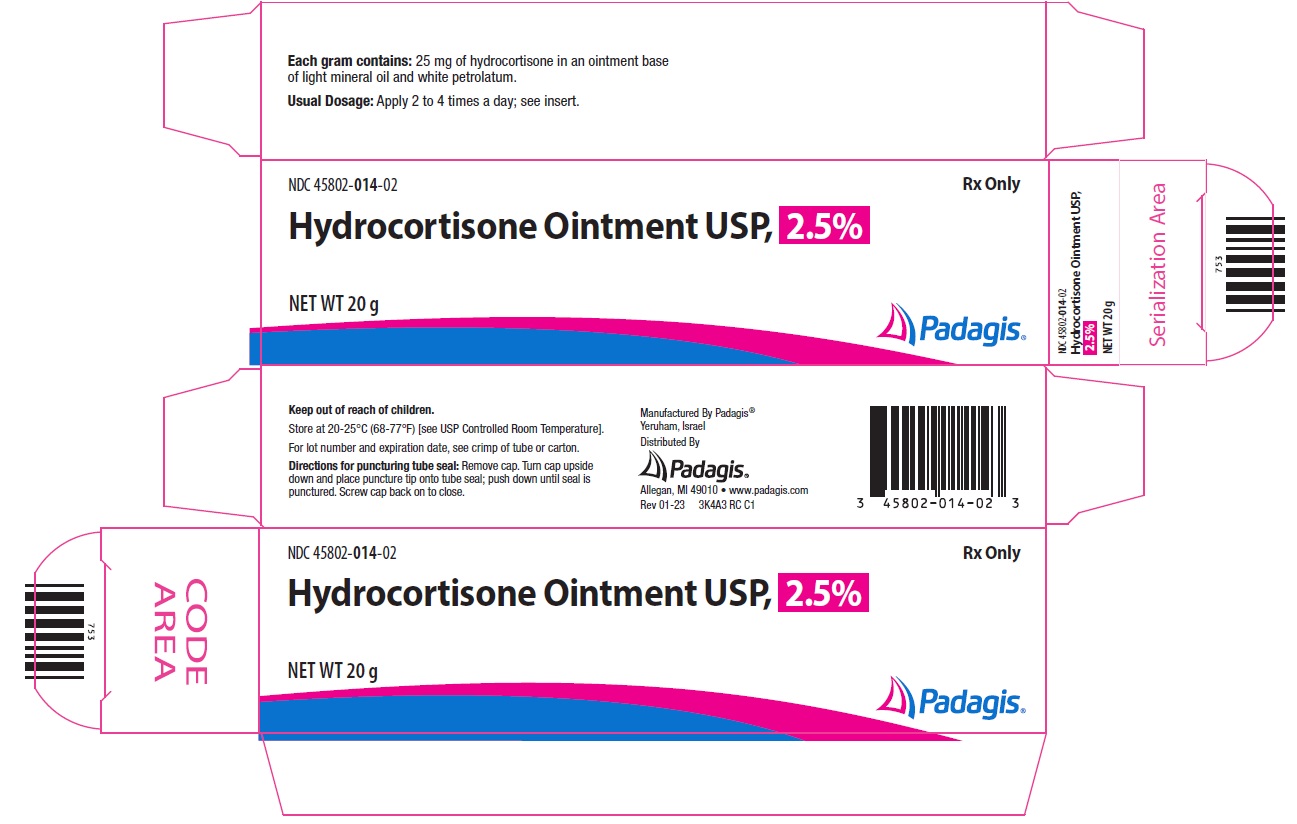
HOW SUPPLIEDHydrocortisone Cream USP, 2.5% is available as follows: 20 g tube (NDC 45802-004-02) 1 oz. (28 g) tube (NDC 45802-004-03) Hydrocortisone Ointment USP, 2.5% is available as follows: 20 g tube (NDC ...
-
STORAGEStore at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Keep out of the reach of children.
-
SPL UNCLASSIFIED SECTIONManufactured By Perrigo plc, Bronx, NY 10457 - Distributed By Padagis - Allegan, MI 49010 - www.padagis.com - Rev 01-22 - 1F300 RC JX2 - Manufactured By Padagis® Yeruham, Israel - Distributed ...
-
Principal Display Panel - Hydrocortisone Cream USP, 2.5% - 28 gNDC 45802-004-03 - Rx Only - Hydrocortisone Cream USP, 2.5% NET WT 28 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug ...
-
Principal Display Panel - Hydrocortisone Ointment USP, 2.5% - 20 gNDC 45802-014-02 - Rx Only - Hydrocortisone Ointment USP, 2.5% NET WT 20 g - The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug ...
-
INGREDIENTS AND APPEARANCEProduct Information