Label: FLUOCINOLONE ACETONIDE oil
- NDC Code(s): 45802-009-10
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FLUOCINOLONE ACETONIDE OIL safely and effectively. See full prescribing information for FLUOCINOLONE ACETONIDE OIL. FLUOCINOLONE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Fluocinolone acetonide oil, 0.01% ear drops is indicated for the topical treatment of chronic eczematous external otitis in adults and pediatric patients 2 years of age and older.
-
2 DOSAGE AND ADMINISTRATION Fluocinolone acetonide oil, 0.01% ear drops is for otic administration only. Not for oral, ophthalmic, or intravaginal use. Apply fluocinolone acetonide oil, 0.01% ear drops into the affected ear ...
-
3 DOSAGE FORMS AND STRENGTHS Ear drops, containing 0.01% fluocinolone acetonide supplied in bottles containing 20 mL (dropper included).
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Endocrine System Adverse Reactions - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in more detail in other sections of the labeling: • Endocrine System Adverse Reactions [see Warnings and Precautions (5.1), Use in Specific ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from case reports, case series, and observational studies on fluocinolone acetonide use in pregnant women have not identified a drug-associated risk ...
-
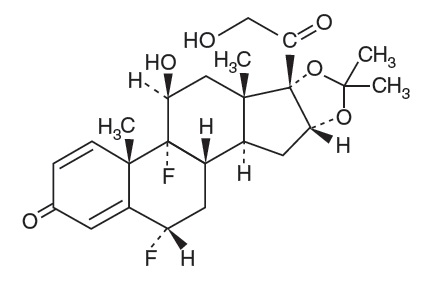
11 DESCRIPTION Fluocinolone Acetonide Oil, 0.01% Ear Drops contains fluocinolone acetonide [(6α, 11β, 16α)-6,9-difluoro-11,21-dihydroxy-16, 17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in eczematous ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, genotoxicity, or fertility studies were conducted with fluocinolone acetonide oil, 0.01% ear drops. However, some ...
-
14 CLINICAL STUDIES In two vehicle-controlled trials (Trial 1 and Trial 2), 154 subjects (adults and pediatric subjects 2 years of age and older) with chronic eczematous external otitis were treated with 5 drops per ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Fluocinolone Acetonide Oil, 0.01% Ear Drops is supplied in bottles containing 20 mL, (dropper included) (NDC 45802-009-10). Storage: Keep tightly closed. Store at 20°-25°C (68°-77°F); excursions ...
-
17 PATIENT COUNSELING INFORMATION Administration Instructions - Advise patients that fluocinolone acetonide oil, 0.01% ear drops is for otic administration only and not for oral, ophthalmic, or intravaginal use [see Dosage and ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 45802-009-10 - Rx Only - Fluocinolone Acetonide Oil, 0.01% (Ear Drops) For Otic Use Only - Not For Ophthalmic Use - NET CONTENTS - 20 mL - The following image is a placeholder representing the product ...
-
INGREDIENTS AND APPEARANCEProduct Information