Label: SUMATRIPTAN SUCCINATE AND NAPROXEN SODIUM tablet
- NDC Code(s): 44183-850-09
- Packager: Currax Pharmaceuticals LLC dba Cypress, Hawthorn, Macoven
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Sumatriptan and Naproxen Sodium Tablets safely and effectively. See full prescribing information for Sumatriptan and Naproxen Sodium ...These highlights do not include all the information needed to use Sumatriptan and Naproxen Sodium Tablets safely and effectively. See full prescribing information for Sumatriptan and Naproxen Sodium Tablets.
Sumatriptan and Naproxen Sodium Tablets, for oral use
Initial U.S. Approval: 2008WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. (5.1)
- Sumatriptan and Naproxen Sodium Tablets is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1)
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events. (5.2)
RECENT MAJOR CHANGES
Warnings and Precautions (5.14) 11/2024 INDICATIONS AND USAGE
Sumatriptan and Naproxen Sodium Tablets is a combination of sumatriptan, a serotonin (5-HT) 1b/1d receptor agonist (triptan), and naproxen sodium, a non-steroidal anti-inflammatory drug, indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years of age and older. (1)
Limitations of Use:
DOSAGE AND ADMINISTRATION
Adults
- Recommended dosage: 1 tablet of 85/500 mg. (2.1)
- Maximum dosage in a 24-hour period: 2 tablets of 85/500 mg; separate doses by at least 2 hours. (2.1)
Pediatric Patients 12 to 17 years of Age
- Recommended dosage: 1 tablet of 10/60 mg. (2.2)
- Maximum dosage in a 24-hour period: 1 tablet of 85/500 mg.
Mild to Moderate Hepatic Impairment
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- History of coronary artery disease or coronary vasospasm. (4)
- In the setting of CABG surgery. (4)
- Wolff-Parkinson-White syndrome or other cardiac accessory conduction pathway disorders. (4)
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine. (4)
- Peripheral vascular disease. (4)
- Ischemic bowel disease. (4)
- Uncontrolled hypertension. (4)
- Recent (within 24 hours) use of another 5-HT1 agonist (e.g., another triptan) or of ergotamine-containing medication. (4)
- Concurrent or recent (past 2 weeks) use of monoamine oxidase-A inhibitor. (4)
- History of asthma, urticaria, other allergic type reactions, rhinitis, or nasal polyps syndrome after taking aspirin or other NSAID/analgesic drugs. (4)
- Known hypersensitivity to sumatriptan, naproxen, or any components of Sumatriptan and Naproxen Sodium Tablets (angioedema and anaphylaxis seen). (4)
- Severe hepatic impairment. (4)
WARNINGS AND PRECAUTIONS
- Cardiovascular Thrombotic Events: Perform cardiac evaluation in patients with cardiovascular risk factors. (5.1)
- Arrhythmias: Discontinue Sumatriptan and Naproxen Sodium Tablets if occurs. (5.3)
- Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure: Generally not associated with myocardial ischemia; evaluate for coronary artery disease in patients at high risk. (5.4)
- Cerebrovascular Events: Discontinue Sumatriptan and Naproxen Sodium Tablets if occurs. (5.5)
- Other Vasospasm Reactions: Discontinue Sumatriptan and Naproxen Sodium Tablets if non-coronary vasospastic reaction occurs. (5.6)
- Hepatotoxicity: Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. (5.7)
- Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. (5.8)
- Heart Failure and Edema: Avoid use of Sumatriptan and Naproxen Sodium Tablets in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure. (5.9)
- Medication Overuse Headache: Detoxification may be necessary. (5.10)
- Serotonin Syndrome: Discontinue Sumatriptan and Naproxen Sodium Tablets if occurs. (5.11)
- Renal Toxicity and Hyperkalemia: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of Sumatriptan and Naproxen Sodium Tablets in patients with advanced renal disease. (5.12)
- Anaphylactic Reactions: Sumatriptan and Naproxen Sodium Tablets should not be given to patients with the aspirin triad. Seek emergency help if an anaphylactic reaction occurs. (5.13)
- Serious Skin Reactions: Discontinue Sumatriptan and Naproxen Sodium Tablets at first sign of rash or other signs of hypersensitivity. (5.14)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue and evaluate clinically. (5.15)
- Fetal Toxicity: Limit use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus. (5.16, 8.1)
- Hematologic Toxicity: Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia. (5.17)
- Exacerbation of Asthma Related to Aspirin Sensitivity: Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity). (5.18)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) were:
- Adults: Dizziness, somnolence, nausea, chest discomfort/chest pain, neck/throat/jaw pain/tightness/pressure, paresthesia, dyspepsia, dry mouth. (6.1)
- Pediatrics: Hot flush (i.e., hot flash[es]) and muscle tightness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Currax Pharmaceuticals LLC at 1-800-793-2145 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs that Interfere with Hemostasis (e.g. warfarin, aspirin, SSRIs/SNRIs): Monitor patients for bleeding who are concomitantly taking Sumatriptan and Naproxen Sodium Tablets with drugs that interfere with hemostasis. Concomitant use of Sumatriptan and Naproxen Sodium Tablets and analgesic doses of aspirin is not generally recommended. (7.1)
- ACE Inhibitors and ARBs: Concomitant use with Sumatriptan and Naproxen Sodium Tablets in elderly, volume depleted, or those with renal impairment may result in deterioration of renal function. In such high risk patients, monitor for signs of worsening renal function. (7.1)
- Diuretics: NSAIDs can reduce natriuretic effect of loop and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects. (7.1)
- Digoxin: Concomitant use with Sumatriptan and Naproxen Sodium Tablets can increase serum concentration and prolong half-life of digoxin. Monitor serum digoxin levels. (7.1)
- Lithium: Increases lithium plasma levels. (7.1)
- Methotrexate: Increases methotrexate plasma levels. (7.1)
USE IN SPECIFIC POPULATIONS
- Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of Sumatriptan and Naproxen Sodium Tablets in women who have difficulties conceiving (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
2.2 Dosage in Pediatric Patients 12 to 17 Years of Age
2.3 Dosing in Patients with Hepatic Impairment
2.4 Administration Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
5.2 Gastrointestinal Bleeding, Ulceration, and Perforation
5.3 Arrhythmias
5.4 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
5.5 Cerebrovascular Events
5.6 Other Vasospasm Reactions
5.7 Hepatotoxicity
5.8 Hypertension
5.9 Heart Failure and Edema
5.10 Medication Overuse Headache
5.11 Serotonin Syndrome
5.12 Renal Toxicity and Hyperkalemia
5.13 Anaphylactic Reactions
5.14 Serious Skin Reactions
5.15 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
5.16 Fetal Toxicity
5.17 Hematologic Toxicity
5.18 Exacerbation of Asthma Related to Aspirin Sensitivity
5.19 Seizures
5.20 Masking of Inflammation and Fever
5.21 Laboratory Monitoring
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Clinically Significant Drug Interactions with Sumatriptan and Naproxen Sodium Tablets
7.2 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adults
14.2 Pediatric Patients 12 to 17 Years of Age
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)Cardiovascular Thrombotic Events - Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke ...
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see Warnings and Precautions (5.1)].
- Sumatriptan and Naproxen Sodium Tablets is contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) Warnings and Precautions (5.1)].
CloseGastrointestinal Bleeding, Ulceration, and Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGESumatriptan and Naproxen Sodium Tablets is indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years of age and older. Limitations of ...
Sumatriptan and Naproxen Sodium Tablets is indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years of age and older.
CloseLimitations of Use:
- Use only if a clear diagnosis of migraine headache has been established. If a patient has no response to the first migraine attack treated with Sumatriptan and Naproxen Sodium Tablets, reconsider the diagnosis of migraine before Sumatriptan and Naproxen Sodium Tablets is administered to treat any subsequent attacks.
- Sumatriptan and Naproxen Sodium Tablets is not indicated for the prevention of migraine attacks.
- Safety and effectiveness of Sumatriptan and Naproxen Sodium Tablets have not been established for cluster headache.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adults - The recommended dosage for adults is 1 tablet of Sumatriptan and Naproxen Sodium Tablets 85/500 mg. Sumatriptan and Naproxen Sodium Tablets 85/500 mg contains a dose of ...
2.1 Dosage in Adults
The recommended dosage for adults is 1 tablet of Sumatriptan and Naproxen Sodium Tablets 85/500 mg. Sumatriptan and Naproxen Sodium Tablets 85/500 mg contains a dose of sumatriptan higher than the lowest effective dose. The choice of the dose of sumatriptan, and of the use of a fixed combination such as in Sumatriptan and Naproxen Sodium Tablets 85/500 mg should be made on an individual basis, weighing the possible benefit of a higher dose of sumatriptan with the potential for a greater risk of adverse reactions.
The maximum recommended dosage in a 24-hour period is 2 tablets, taken at least 2 hours apart.
The safety of treating an average of more than 5 migraine headaches in adults in a 30-day period has not been established.
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
2.2 Dosage in Pediatric Patients 12 to 17 Years of Age
The recommended dosage for pediatric patients 12 to 17 years of age is 1 tablet of Sumatriptan and Naproxen Sodium Tablets 10/60 mg.
The maximum recommended dosage in a 24-hour period is 1 tablet of Sumatriptan and Naproxen Sodium Tablets 85/500 mg.
The safety of treating an average of more than 2 migraine headaches in pediatric patients in a 30-day period has not been established.
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
2.3 Dosing in Patients with Hepatic Impairment
Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with severe hepatic impairment [see Contraindications (4), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
In patients with mild to moderate hepatic impairment, the recommended dosage in a 24-hour period is 1 tablet of Sumatriptan and Naproxen Sodium Tablets 10/60 mg [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
Close2.4 Administration Information
Sumatriptan and Naproxen Sodium Tablets may be administered with or without food. Tablets should not be split, crushed, or chewed.
-
3 DOSAGE FORMS AND STRENGTHS10 mg sumatriptan/60 mg naproxen sodium, light-blue film-coated tablets, debossed on one side with "TREXIMET" and the other side with "10-60". 85 mg sumatriptan/500 mg naproxen sodium, blue ...
10 mg sumatriptan/60 mg naproxen sodium, light-blue film-coated tablets, debossed on one side with "TREXIMET" and the other side with "10-60".
85 mg sumatriptan/500 mg naproxen sodium, blue film-coated tablets, debossed on one side with "TREXIMET".
Close -
4 CONTRAINDICATIONSSumatriptan and Naproxen Sodium Tablets is contraindicated in the following patients: Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented ...
Sumatriptan and Naproxen Sodium Tablets is contraindicated in the following patients:
- Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal's angina [see Warnings and Precautions (5.1)].
- In the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)].
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.3)].
- History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.5)].
- Peripheral vascular disease [see Warnings and Precautions (5.6)].
- Ischemic bowel disease [see Warnings and Precautions (5.6)].
- Uncontrolled hypertension [see Warnings and Precautions (5.8)].
- Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine1 (5-HT1) agonist [see Drug Interactions (7)].
- Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (7), Clinical Pharmacology (12.3)].
- History of asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.13, 5.14, 5.18)].
- Known hypersensitivity (e.g., anaphylactic reactions, angioedema, and serious skin reactions) to sumatriptan, naproxen, or any components of Sumatriptan and Naproxen Sodium Tablets [see Warnings and Precautions (5.14)].
- Severe hepatic impairment [see Warnings and Precautions (5.7), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Thrombotic Events - The use of Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD) and in the ...
5.1 Cardiovascular Thrombotic Events
The use of Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD) and in the setting of coronary artery bypass graft (CABG) surgery due to increased risk of serious cardiovascular events with sumatriptan and NSAIDS [see Contraindications (4)].
Cardiovascular Events with Sumatriptan
There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan. Some of these reactions occurred in patients without known CAD. Sumatriptan and Naproxen Sodium Tablets may cause coronary artery vasospasm (Prinzmetal's angina), even in patients without a history of CAD.
Cardiovascular Thrombotic Events with Nonsteroidal Anti-inflammatory Drugs
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as naproxen, increases the risk of serious gastrointestinal (GI) events [see Warnings and Precautions (5.2)].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG [see Contraindications (4)].
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post-MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Perform a cardiovascular evaluation in patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving Sumatriptan and Naproxen Sodium Tablets. If there is evidence of CAD or coronary artery vasospasm, Sumatriptan and Naproxen Sodium Tablets is contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of Sumatriptan and Naproxen Sodium Tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of Sumatriptan and Naproxen Sodium Tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of Sumatriptan and Naproxen Sodium Tablets.
Physicians and patients should remain alert for the development of cardiovascular events, even in the absence of previous cardiovascular symptoms. Patients should be informed about the signs and/or symptoms of serious cardiovascular events and the steps to take if they occur.
5.2 Gastrointestinal Bleeding, Ulceration, and Perforation
NSAIDs, including naproxen, a component of Sumatriptan and Naproxen Sodium Tablets, cause serious gastrointestinal adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only 1 in 5 patients who develop a serious upper gastrointestinal adverse event on NSAID therapy is symptomatic. Upper gastrointestinal ulcers, gross bleeding, or perforation caused by NSAIDs appear to occur in approximately 1% of patients treated daily for 3 to 6 months and in about 2% to 4% of patients treated for 1 year. However, even short-term therapy is not without risk.
Among 3,302 adult patients with migraine who received Sumatriptan and Naproxen Sodium Tablets in controlled and uncontrolled clinical trials, 1 patient experienced a recurrence of gastric ulcer after taking 8 doses over 3 weeks, and 1 patient developed a gastric ulcer after treating an average of 8 attacks per month over 7 months.
Risk Factors for GI Bleeding, Ulceration, and Perforation
Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing gastrointestinal bleeding compared with patients with neither of these risk factors. Other factors that increase the risk for gastrointestinal bleeding in patients treated with NSAIDs include longer duration of NSAID therapy; concomitant use of oral corticosteroids, aspirin, anticoagulants, or selective serotonin reuptake inhibitors (SSRIs); smoking; use of alcohol; older age; and poor general health status. Most postmarketing reports of fatal gastrointestinal events occurred in elderly or debilitated patients, and therefore special care should be taken in treating this population. Additionally, patients with advanced liver disease and/or coagulopathy are at increased risk for GI bleeding.
Strategies to Minimize the GI Risks in NSAID-treated patients:
- Use the lowest effective dosage for the shortest possible duration.
- Avoid administration of more than one NSAID at a time.
- Avoid use in patients at higher risk unless benefits are expected to outweigh the increased risk of bleeding. For high risk patients, as well as those with active GI bleeding, consider alternate therapies other than NSAIDs.
- Remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy.
- If a serious GI adverse event is suspected, promptly initiate evaluation and treatment, and discontinue Sumatriptan and Naproxen Sodium Tablets until a serious GI adverse event is ruled out.
- In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, monitor patients more closely for evidence of GI bleeding [see Drug Interactions (7)].
5.3 Arrhythmias
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue Sumatriptan and Naproxen Sodium Tablets if these disturbances occur. Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
5.4 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
Sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with sumatriptan and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with CAD and those with Prinzmetal's variant angina.
5.5 Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue Sumatriptan and Naproxen Sodium Tablets if a cerebrovascular event occurs.
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with a history of stroke or TIA [see Contraindications (4)].
5.6 Other Vasospasm Reactions
Sumatriptan may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud's syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist, rule out a vasospastic reaction before receiving additional Sumatriptan and Naproxen Sodium Tablets.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.
5.7 Hepatotoxicity
Borderline elevations of 1 or more liver tests may occur in up to 15% of patients who take NSAIDs including naproxen, a component of Sumatriptan and Naproxen Sodium Tablets. Hepatic abnormalities may be the result of hypersensitivity rather than direct toxicity. These abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. Notable (3 times the upper limit of normal) elevations of SGPT (ALT) or SGOT (AST) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare, sometimes fatal cases of severe hepatic injury, including jaundice and fatal fulminant hepatitis, liver necrosis, and hepatic failure have been reported with NSAIDs.
Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with severe hepatic impairment [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)]. A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with Sumatriptan and Naproxen Sodium Tablets. Sumatriptan and Naproxen Sodium Tablets should be discontinued if clinical signs and symptoms consistent with liver disease develop, if systemic manifestations occur (e.g., eosinophilia, rash), or if abnormal liver tests persist or worsen.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flulike" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue Sumatriptan and Naproxen Sodium Tablets immediately, and perform a clinical evaluation of the patient.
5.8 Hypertension
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1 agonists, including sumatriptan, a component of Sumatriptan and Naproxen Sodium Tablets. This occurrence has included patients without a history of hypertension.
NSAIDs, including naproxen, a component of Sumatriptan and Naproxen Sodium Tablets, can also lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of cardiovascular events. Patients taking angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs [see Drug Interactions (7)].
Monitor blood pressure in patients treated with Sumatriptan and Naproxen Sodium Tablets. Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)].
5.9 Heart Failure and Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of naproxen may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) [see Drug Interactions (7)].
Avoid the use of Sumatriptan and Naproxen Sodium Tablets in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If Sumatriptan and Naproxen Sodium Tablets is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Since each Sumatriptan and Naproxen Sodium Tablets 85/500 mg tablet contains approximately 60 mg of sodium and each Sumatriptan and Naproxen Sodium Tablets 10/60 mg tablet contains approximately 20 mg of sodium, this should be considered in patients whose overall intake of sodium must be severely restricted.
5.10 Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.11 Serotonin Syndrome
Serotonin syndrome may occur with Sumatriptan and Naproxen Sodium Tablets, particularly during coadministration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Contraindications (4) and Drug Interactions (7.1)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue Sumatriptan and Naproxen Sodium Tablets if serotonin syndrome is suspected.
5.12 Renal Toxicity and Hyperkalemia
Renal Toxicity
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, salt depletion, those taking diuretics and angiotensin-converting enzyme (ACE) inhibitors or ARBs, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Sumatriptan and Naproxen Sodium Tablets should be discontinued if clinical signs and symptoms consistent with renal disease develop or if systemic manifestations occur.
Sumatriptan and Naproxen Sodium Tablets is not recommended for use in patients with severe renal impairment (creatinine clearance [CrCl] <30 mL/min) unless the benefits are expected to outweigh the risk of worsening renal function [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. If Sumatriptan and Naproxen Sodium Tablets is used in patients with advanced renal disease, monitor patients for signs of worsening renal function. Monitor renal function in patients with mild (CrCl = 60 to 89 mL/min) or moderate (CrCl = 30 to 59 mL/min) renal impairment, preexisting kidney disease, or dehydration.
The renal effects of Sumatriptan and Naproxen Sodium Tablets may hasten the progression of renal dysfunction in patients with pre-existing renal disease.
Correct volume status in dehydrated or hypovolemic patients prior to initiating Sumatriptan and Naproxen Sodium Tablets. Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia during use of Sumatriptan and Naproxen Sodium Tablets [see Drug Interactions (7)]. Avoid the use of Sumatriptan and Naproxen Sodium Tablets in patients with advanced renal disease unless the benefits are expected to outweigh the risk of worsening renal function. If Sumatriptan and Naproxen Sodium Tablets is used in patients with advanced renal disease, monitor patients for signs of worsening renal function.
5.13 Anaphylactic Reactions
Anaphylactic reactions may occur in patients without known prior exposure to either component of Sumatriptan and Naproxen Sodium Tablets. Such reactions can be life-threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens although anaphylactic reactions with naproxen have occurred in patient without known hypersensitivity to naproxen or to patients with aspirin sensitive asthma [see Contraindications (4) and Warnings and Precautions (5.18)]. Sumatriptan and Naproxen Sodium Tablets should not be given to patients with the aspirin triad. This symptom complex typically occurs in patients with asthma who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs [see Contraindications (4)].
Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with a history of hypersensitivity reaction to sumatriptan, naproxen, or any other component of Sumatriptan and Naproxen Sodium Tablets. Naproxen has been associated with anaphylactic reactions in patients without known hypersensitivity to naproxen and in patients with aspirin-sensitive asthma [see Contraindications (4) and Warnings and Precautions (5.18)]. Seek emergency help if an anaphylactic reaction occurs.
5.14 Serious Skin Reactions
NSAID-containing products can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions and to discontinue the use of Sumatriptan and Naproxen Sodium Tablets at the first appearance of skin rash or any other sign of hypersensitivity. Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with previous serious skin reactions to NSAIDs [see Contraindications (4)].
5.15 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as Sumatriptan and Naproxen Sodium Tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue Sumatriptan and Naproxen Sodium Tablets and evaluate the patient immediately.
5.16 Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus
Avoid use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, in pregnant women at about 30 weeks gestation and later. NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit Sumatriptan and Naproxen Sodium Tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if Sumatriptan and Naproxen Sodium Tablets treatment extends beyond 48 hours. Discontinue Sumatriptan and Naproxen Sodium Tablets if oligohydramnios occurs and follow up according to clinical practice [see Use in Specific Populations (8.1)].
5.17 Hematologic Toxicity
Anemia has occurred in patients receiving NSAIDs. This may be due to fluid retention, occult or gross gastrointestinal blood loss, or an incompletely described effect upon erythropoiesis. If a patient treated with Sumatriptan and Naproxen Sodium Tablets has signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders or concomitant use of warfarin, other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding [see Drug Interactions (7)].
5.18 Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma [see Contraindications (4)].
When Sumatriptan and Naproxen Sodium Tablets is used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
5.19 Seizures
Seizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan and Naproxen Sodium Tablets should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
5.20 Masking of Inflammation and Fever
The pharmacological activity of Sumatriptan and Naproxen Sodium Tablets in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
Close5.21 Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and a chemistry profile periodically [see Warnings and Precautions (5.2, 5.7, 5.12)].
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in labeling: Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)] GI Bleeding, Ulceration and ...
The following serious adverse reactions are described below and elsewhere in labeling:
- Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)]
- GI Bleeding, Ulceration and Perforation [see Warnings and Precautions (5.2)]
- Arrhythmias [see Warnings and Precautions (5.3)]
- Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure [see Warnings and Precautions (5.4)]
- Cerebrovascular Events [see Warnings and Precautions (5.5)]
- Other Vasospasm Reactions [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
- Hypertension [see Warnings and Precautions (5.8)]
- Heart Failure and Edema [see Warnings and Precautions (5.9)]
- Medication Overuse Headache [see Warnings and Precautions (5.10)]
- Serotonin Syndrome [see Warnings and Precautions (5.11)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.12)]
- Anaphylactic Reactions [see Warnings and Precautions (5.13)]
- Serious Skin Reactions [see Warnings and Precautions (5.14)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.15)]
- Hematological Toxicity [see Warnings and Precautions (5.17)]
- Exacerbation Asthma Related to Aspirin Sensitivity [see Warnings and Precautions (5.18)]
- Seizures [see Warnings and Precautions (5.19)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The adverse reactions reported below are specific to the clinical trials with Sumatriptan and Naproxen Sodium Tablets 85/500 mg. See also the full prescribing information for naproxen and sumatriptan products.
Table 1 lists adverse reactions that occurred in 2 placebo-controlled clinical trials (Study 1 and 2) in adult patients who received 1 dose of study drug. Only adverse reactions that occurred at a frequency of 2% or more in any group treated with Sumatriptan and Naproxen Sodium Tablets 85/500 mg and that occurred at a frequency greater than the placebo group are included in Table 1.
Table 1. Adverse Reactions in Pooled Placebo-Controlled Trials in Adult Patients with Migraine Adverse Reactions Sumatriptan and Naproxen Sodium Tablets 85/500 mg
%
(n = 737)Placebo
%
(n = 752)Sumatriptan
85 mg
%
(n = 735)Naproxen Sodium
500 mg
%
(n = 732)Nervous system disorders Dizziness 4 2 2 2 Somnolence 3 2 2 2 Paresthesia 2 <1 2 <1 Gastrointestinal disorders Nausea 3 1 3 <1 Dyspepsia 2 1 2 1 Dry mouth 2 1 2 <1 Pain and other pressure sensations Chest discomfort/chest pain 3 <1 2 1 Neck/throat/jaw pain/tightness/pressure 3 1 3 1 The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Pediatric Patients 12 to 17 Years of Age
In a placebo-controlled clinical trial that evaluated pediatric patients 12 to 17 years of age who received 1 dose of Sumatriptan and Naproxen Sodium Tablets 10/60 mg, 30/180 mg, or 85/500 mg, adverse reactions occurred in 13% of patients who received 10/60 mg, 9% of patients who received 30/180 mg, 13% who received 85/500 mg, and 8% who received placebo. No patients who received Sumatriptan and Naproxen Sodium Tablets experienced adverse reactions leading to withdrawal from the trial. The incidence of adverse reactions in pediatric patients 12 to 17 years of age was comparable across all 3 doses compared with placebo. Table 2 lists adverse reactions that occurred in a placebo-controlled trial in pediatric patients 12 to 17 years of age at a frequency of 2% or more with Sumatriptan and Naproxen Sodium Tablets and were more frequent than the placebo group.
Table 2. Adverse Reactions in a Placebo-Controlled Trial in Pediatric Patients 12 to 17 Years of Age with Migraine Adverse Reactions Sumatriptan and Naproxen Sodium Tablets 10/60 mg
%Sumatriptan and Naproxen Sodium Tablets 30/180 mg
%Sumatriptan and Naproxen Sodium Tablets 85/500 mg
%Placebo
%(n = 96) (n = 97) (n = 152) (n = 145) Vascular Hot flush (i.e., hot flash[es]) 0 2 <1 0 Musculoskeletal Muscle tightness 0 0 2 0 Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NSAIDs, such as naproxen, which is a component of Sumatriptan and Naproxen Sodium Tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Appendages: exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and fixed drug eruption (FDE) [see Warnings and Precautions (5.14)].
-
7 DRUG INTERACTIONS7.1 Clinically Significant Drug Interactions with Sumatriptan and Naproxen Sodium Tablets - See Table 3 for clinically significant drug interactions with NSAIDs or Sumatriptan. Table 3 ...
7.1 Clinically Significant Drug Interactions with Sumatriptan and Naproxen Sodium Tablets
See Table 3 for clinically significant drug interactions with NSAIDs or Sumatriptan.
Table 3. Clinically Significant Drug Interactions with Naproxen or Sumatriptan Ergot-Containing Drugs Clinical Impact: Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Intervention: Because these effects may be additive, coadministration of Sumatriptan and Naproxen Sodium Tablets and ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) within 24 hours of each other is contraindicated. Monoamine Oxidase-A Inhibitors Clinical Impact: MAO-A inhibitors increase systemic exposure of orally administered sumatriptan by 7-fold. Intervention: The use of Sumatriptan and Naproxen Sodium Tablets in patients receiving MAO-A inhibitors is contraindicated. Other 5-HT1 Agonists Clinical Impact: 5-HT1 agonist drugs can cause vasospastic effects. Intervention: Because these effects may be additive, coadministration of Sumatriptan and Naproxen Sodium Tablets and other 5 HT1 agonists (e.g., triptans) within 24 hours of each other is contraindicated. Drugs That Interfere with Hemostasis Clinical Impact: - Naproxen and anticoagulants such as warfarin have a synergistic effect on bleeding. The concomitant use of naproxen and anticoagulants have an increased risk of serious bleeding compared to the use of either drug alone.
- Serotonin release by platelets plays an important role in hemostasis. Case-control and cohort epidemiological studies showed that concomitant use of drugs that interfere with serotonin reuptake and an NSAID may potentiate the risk of bleeding more than an NSAID alone.
Intervention: Monitor patients with concomitant use of Sumatriptan and Naproxen Sodium Tablets with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.17)]. Aspirin Clinical Impact: A pharmacodynamic (PD) study has demonstrated an interaction in which lower dose naproxen (220mg/day or 220mg twice daily) interfered with the antiplatelet effect of low-dose immediate-release aspirin, with the interaction most marked during the washout period of naproxen [see Clinical Pharmacology (12.2)]. There is reason to expect that the interaction would be present with prescription doses of naproxen or with enteric-coated low-dose aspirin; however, the peak interference with aspirin function may be later than observed in the PD study due to the longer washout period.
Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].Intervention: Because there may be an increased risk of cardiovascular events following discontinuation of naproxen due to the interference with the antiplatelet effect of aspirin during the washout period, for patients taking low-dose aspirin for cardioprotection who require intermittent analgesics, consider use of an NSAID that does not interfere with the antiplatelet effect of aspirin, or non-NSAID analgesics where appropriate.
Concomitant use of Sumatriptan and Naproxen Sodium Tablets and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding [see Warnings and Precautions (5.17)].Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome Clinical Impact: Cases of serotonin syndrome have been reported during coadministration of triptans and SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.11)]. Intervention: Discontinue Sumatriptan and Naproxen Sodium Tablets if serotonin syndrome is suspected. ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-blockers Clinical Impact: - NSAIDs may diminish the antihypertensive effect of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers (including propranolol).
- In patients who are elderly, volume-depleted (including those on diuretic therapy), or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible.
Intervention: - During concomitant use of Sumatriptan and Naproxen Sodium Tablets and ACE-inhibitors, ARBs, or beta-blockers, monitor blood pressure to ensure that the desired blood pressure is obtained [see Warnings and Precautions (5.8)].
- During concomitant use of Sumatriptan and Naproxen Sodium Tablets and ACE-inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor for signs of worsening renal function [see Warnings and Precautions (5.8)].
Diuretics Clinical Impact: Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.8, 5.12)]. Digoxin Clinical Impact: The concomitant use of naproxen with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets and digoxin, monitor serum digoxin levels. Lithium Clinical Impact: NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets and lithium, monitor patients for signs of lithium toxicity. Methotrexate Clinical Impact: Concomitant administration of some NSAIDs with high-dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and gastrointestinal toxicity. Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets and methotrexate, monitor patients for methotrexate toxicity. Cyclosporine Clinical Impact: Concomitant use of NSAIDs and cyclosporine may increase cyclosporine's nephrotoxicity. Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets and cyclosporine, monitor patients for signs of worsening renal function. NSAIDs and Salicylates Clinical Impact: Concomitant use of naproxen with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.2)]. Intervention: The concomitant use of naproxen with other NSAIDs or salicylates is not recommended. Pemetrexed Clinical Impact: Concomitant use of NSAIDs and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). Intervention: During concomitant use of Sumatriptan and Naproxen Sodium Tablets and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed.
In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration.Probenecid Clinical Impact: Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly. The clinical significance of this is unknown. Intervention: Reduce the frequency of administration of Sumatriptan and Naproxen Sodium Tablets when given concurrently with probenecid. Close7.2 Drug/Laboratory Test Interactions
Blood Tests
Naproxen may decrease platelet aggregation and prolong bleeding time. This effect should be kept in mind when bleeding times are determined.
Urine Tests
The administration of naproxen sodium may result in increased urinary values for 17-ketogenic steroids because of an interaction between the drug and/or its metabolites with m-di-nitrobenzene used in this assay. Although 17-hydroxy-corticosteroid measurements (Porter-Silber test) do not appear to be artificially altered, it is suggested that therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used.
Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA).
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading ...
8.1 Pregnancy
Risk Summary
Use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of Sumatriptan and Naproxen Sodium Tablets use between about 20 and 30 weeks of gestation, and avoid Sumatriptan and Naproxen Sodium Tablets use at about 30 weeks of gestation and later in pregnancy (see Clinical Considerations, Data).
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive.
Data from a prospective pregnancy exposure registry and epidemiological studies of pregnant women have not detected an increased frequency of birth defects or a consistent pattern of birth defects among women exposed to sumatriptan compared with the general population (see Human Data). In animal studies, administration of sumatriptan and naproxen, alone or in combination, during pregnancy resulted in developmental toxicity (increased incidences of fetal malformations, embryofetal and pup mortality, decreased embryofetal growth) at clinically relevant doses (see Animal Data). Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as naproxen sodium resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The reported rate of major birth defects among deliveries to women with migraine ranged from 2.2% to 2.9% and the reported rate of miscarriage was 17%, which were similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Several studies have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, can cause premature closure of the fetal ductus arteriosus (see Data).
Oligohydramnios/Neonatal Renal Impairment:
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If Sumatriptan and Naproxen Sodium Tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue Sumatriptan and Naproxen Sodium Tablets and follow up according to clinical practice (see Data).
Data
Human Data
There is some evidence to suggest that when inhibitors of prostaglandin synthesis are used to delay preterm labor, there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus, and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction, and abnormal prostaglandin E levels in preterm infants.
The Sumatriptan/Naratriptan/Treximet (sumatriptan and naproxen sodium) Pregnancy Registry, a population-based international prospective study, collected data for sumatriptan from January 1996 to September 2012. The Registry included only 6 pregnancy exposures to Sumatriptan and Naproxen Sodium Tablets, with no major birth defects reported. The Registry documented outcomes of 626 infants and fetuses exposed to sumatriptan during pregnancy (528 with earliest exposure during the first trimester, 78 during the second trimester, 16 during the third trimester, and 4 unknown). The occurrence of major birth defects (excluding fetal deaths and induced abortions without reported defects and all spontaneous pregnancy losses) during first-trimester exposure to sumatriptan was 4.2% (20/478 [95% CI: 2.6% to 6.5%]) and during any trimester of exposure was 4.2% (24/576 [95% CI: 2.7% to 6.2%]). The sample size in this study had 80% power to detect at least a 1.73- to 1.91-fold increase in the rate of major malformations. The number of exposed pregnancy outcomes accumulated during the registry was insufficient to support definitive conclusions about overall malformation risk or to support making comparisons of the frequencies of specific birth defects. Of the 20 infants with reported birth defects after exposure to sumatriptan in the first trimester, 4 infants had ventricular septal defects, including one infant who was exposed to both sumatriptan and naratriptan, and 3 infants had pyloric stenosis. No other birth defect was reported for more than 2 infants in this group.
In a study using data from the Swedish Medical Birth Register, live births to women who reported using triptans or ergots during pregnancy were compared with those of women who did not. Of the 2,257 births with first-trimester exposure to sumatriptan, 107 infants were born with malformations (relative risk 0.99 [95% CI: 0.91 to 1.21]). A study using linked data from the Medical Birth Registry of Norway to the Norwegian Prescription Database compared pregnancy outcomes in women who redeemed prescriptions for triptans during pregnancy, as well as a migraine disease comparison group who redeemed prescriptions for sumatriptan before pregnancy only, compared with a population control group. Of the 415 women who redeemed prescriptions for sumatriptan during the first trimester, 15 had infants with major congenital malformations (OR 1.16 [95% CI: 0.69 to 1.94]) while for the 364 women who redeemed prescriptions for sumatriptan before, but not during, pregnancy, 20 had infants with major congenital malformations (OR 1.83 [95% CI: 1.17 to 2.88]), each compared with the population comparison group. Additional smaller observational studies evaluating use of sumatriptan during pregnancy have not suggested an increased risk of teratogenicity.
Premature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Animal Data
Oral administration of sumatriptan alone to pregnant rats during the period of organogenesis resulted in an increased incidence of fetal blood vessel (cervicothoracic and umbilical) abnormalities. The highest no-effect dose for embryofetal developmental toxicity in rats was 60 mg/kg/day, or approximately 3 times the maximum recommended human dose (MRHD) of 170 mg/day on a mg/m2 basis.
Oral administration of sumatriptan alone to pregnant rabbits during the period of organogenesis resulted in increased incidences of embryolethality and fetal cervicothoracic vascular and skeletal abnormalities. Intravenous administration of sumatriptan to pregnant rabbits during the period of organogenesis resulted in an increased incidence of embryolethality. The highest oral and intravenous no-effect doses for developmental toxicity in rabbits were 15 (approximately 2 times the MRHD on a mg/m2 basis) and 0.75 mg/kg/day, respectively.
Oral administration of sumatriptan combined with naproxen sodium (5/9, 25/45, or 50/90 mg/kg/day sumatriptan/naproxen sodium) or each drug alone (50/0, 0/90 mg/kg/day sumatriptan/naproxen sodium) to pregnant rabbits during the period of organogenesis resulted in increased total incidences of fetal abnormalities at all doses and increased incidences of specific malformations (cardiac interventricular septal defect in the 50/90 mg/kg/day group, fused caudal vertebrae in the 50/0 and 0/90 mg/kg/day groups) and variations (absent intermediate lobe of the lung, irregular ossification of the skull, incompletely ossified sternal centra) at the highest dose of sumatriptan and naproxen alone and in combination. A no-effect dose for developmental toxicity in rabbit was not established. The lowest effect dose of 5/9 mg/kg/day sumatriptan/naproxen sodium was associated with plasma exposures (AUC) to sumatriptan and naproxen that were less than those attained at the MRHD of 170 mg sumatriptan and 1000 mg naproxen sodium (two tablets of Sumatriptan and Naproxen Sodium Tablets 85/500 mg in a 24-hour period).
Oral administration of sumatriptan alone to rats prior to and throughout gestation resulted in embryofetal toxicity (decreased body weight, decreased ossification, increased incidence of skeletal abnormalities). The highest no-effect dose was 50 mg/kg/day, or approximately 3 times the MRHD on a mg/m2 basis. In offspring of pregnant rats treated orally with sumatriptan during organogenesis, there was a decrease in pup survival. The highest no-effect dose for this effect was 60 mg/kg/day, or approximately 3 times the MRHD on a mg/m2 basis. Oral treatment of pregnant rats with sumatriptan during the latter part of gestation and throughout lactation resulted in a decrease in pup survival. The highest no-effect dose for this finding was 100 mg/kg/day, or approximately 6 times the MRHD on a mg/m2 basis.
In reproduction studies of naproxen in rats (20 mg/kg/day), rabbits (20 mg/kg/day, and mice (170 mg/kg/day, no evidence of impaired fertility or harm to the fetus was observed. The doses tested in rats, rabbits, and mice were less (≤0.8 times) the MRHD, based on body surface area (mg/m2) comparisons.
8.2 Lactation
Risk Summary
The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma. Sumatriptan is excreted in human milk following subcutaneous administration (see Data). There is no information regarding sumatriptan concentrations in milk from lactating women following administration of sumatriptan tablets.
There are no data on the effects of naproxen or sumatriptan on the breastfed infant or the effects of naproxen or sumatriptan on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Sumatriptan and Naproxen Sodium Tablets and any potential adverse effects on the breastfed infant from Sumatriptan and Naproxen Sodium Tablets or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including naproxen tablets, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAIDs, including naproxen tablets, in women who have difficulties conceiving or who are undergoing investigation of infertility.
8.4 Pediatric Use
Safety and effectiveness of Sumatriptan and Naproxen Sodium Tablets in pediatric patients under 12 years of age have not been established.
The safety and efficacy of Sumatriptan and Naproxen Sodium Tablets for the acute treatment of migraine in pediatric patients 12 to 17 years of age was established in a double-blind, placebo-controlled trial [see Adverse Reactions (6.1) and Clinical Studies (14.2)].
8.5 Geriatric Use
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. Sumatriptan and Naproxen Sodium Tablets is not recommended for use in elderly patients who have decreased renal function, higher risk for unrecognized CAD, and increases in blood pressure that may be more pronounced in the elderly [see Warnings and Precautions (5.1, 5.2, 5.3, 5.8, 5.12) and Clinical Pharmacology (12.3)].
A cardiovascular evaluation is recommended for geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving Sumatriptan and Naproxen Sodium Tablets [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Sumatriptan and Naproxen Sodium Tablets is not recommended for use in patients with creatinine clearance less than 30 mL/min. Monitor the serum creatinine or creatinine clearance in patients with mild (CrCl = 60 to 89 mL/min) or moderate (CrCL = 30 to 59 mL/min) renal impairment, preexisting kidney disease, or dehydration [see Warnings and Precautions (5.12) and Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
Sumatriptan and Naproxen Sodium Tablets is contraindicated in patients with severe hepatic impairment. For patients with mild or moderate hepatic impairment, the Sumatriptan and Naproxen Sodium Tablets dose should be reduced. [see Contraindications (4), Warnings and Precautions (5.7), and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEPatients (N = 670) have received single oral doses of 140 to 300 mg of sumatriptan without significant adverse effects. Volunteers (N = 174) have received single oral doses of 140 to 400 mg ...
Patients (N = 670) have received single oral doses of 140 to 300 mg of sumatriptan without significant adverse effects. Volunteers (N = 174) have received single oral doses of 140 to 400 mg without serious adverse events.
Overdose of sumatriptan in animals has been fatal and has been heralded by convulsions, tremor, paralysis, inactivity, ptosis, erythema of the extremities, abnormal respiration, cyanosis, ataxia, mydriasis, salivation, and lacrimation.
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting and epigastric pain. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [see Warnings and Precautions (5.1, 5.2)].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Consider emesis and/or activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Hemodialysis does not decrease the plasma concentration of naproxen because of the high degree of its protein binding. It is unknown what effect hemodialysis or peritoneal dialysis has on the serum concentrations of sumatriptan. Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
Close -
11 DESCRIPTIONSumatriptan and Naproxen Sodium Tablets contains sumatriptan (as the succinate), a selective 5-hydroxytryptamine1 (5-HT1) receptor subtype agonist, and naproxen sodium, a member of the arylacetic ...
Sumatriptan and Naproxen Sodium Tablets contains sumatriptan (as the succinate), a selective 5-hydroxytryptamine1 (5-HT1) receptor subtype agonist, and naproxen sodium, a member of the arylacetic acid group of NSAIDs.
Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
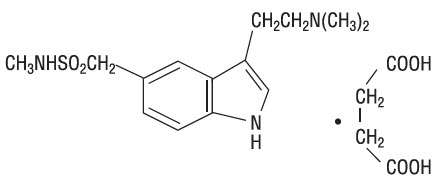
The empirical formula is C14H21N3O2S∙C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
Naproxen sodium is chemically designated as (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid, sodium salt, and it has the following structure:
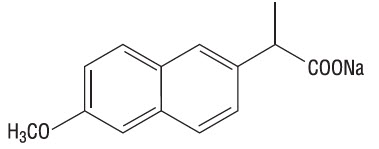
The empirical formula is C14H13NaO3, representing a molecular weight of 252.23. Naproxen sodium is a white-to-creamy white crystalline solid, freely soluble in water at neutral pH.
Each Sumatriptan and Naproxen Sodium Tablets 85/500 mg tablet for oral administration contains 119 mg of sumatriptan succinate equivalent to 85 mg of sumatriptan and 500 mg of naproxen sodium. Each tablet also contains the inactive ingredients croscarmellose sodium, dibasic calcium phosphate, FD&C Blue No. 2, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, sodium bicarbonate, talc, titanium dioxide, and triacetin.
Each Sumatriptan and Naproxen Sodium Tablets 10/60 mg tablet for oral administration contains 14 mg of sumatriptan succinate equivalent to 10 mg of sumatriptan and 60 mg of naproxen sodium. Each tablet also contains the inactive ingredients croscarmellose sodium, dibasic calcium phosphate, FD&C Blue No. 2, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, sodium bicarbonate, talc, and titanium dioxide.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sumatriptan and Naproxen Sodium Tablets contains sumatriptan and naproxen. Sumatriptan binds with high affinity to cloned 5-HT1B/1D receptors. Sumatriptan presumably ...
12.1 Mechanism of Action
Sumatriptan and Naproxen Sodium Tablets contains sumatriptan and naproxen.
Sumatriptan binds with high affinity to cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache through agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of neuropeptide release.
Sumatriptan and Naproxen Sodium Tablets has analgesic, anti-inflammatory, and antipyretic properties. The mechanism of action of Sumatriptan and Naproxen Sodium Tablets, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Naproxen is a potent inhibitor of prostaglandin synthesis in vitro. Naproxen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because naproxen is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.2 Pharmacodynamics
In a healthy volunteer study, 10 days of concomitant administration of naproxen 220 mg once-daily with low-dose immediate-release aspirin (81 mg) showed an interaction with the antiplatelet activity of aspirin as measured by % serum thromboxane B2 inhibition at 24 hours following the day 10 dose [98.7% (aspirin alone) vs 93.1% (naproxen and aspirin)]. The interaction was observed even following discontinuation of naproxen on day 11 (while aspirin dose was continued) but normalized by day 13. In the same study, the interaction was greater when naproxen was administered 30 minutes prior to aspirin [98.7% vs 87.7%] and minimal when aspirin was administered 30 minutes prior to naproxen [98.7% vs 95.4%].
Following administration of naproxen 220 mg twice-daily with low-dose immediate–release aspirin (first naproxen dose given 30 minutes prior to aspirin), the interaction was minimal at 24 h following day 10 dose [98.7% vs 95.7%]. However, the interaction was more prominent after discontinuation of naproxen (washout) on day 11 [98.7% vs 84.3%] and did not normalize completely by day 13 [98.5% vs 90.7%] [see Drug Interactions (7.1)].
Blood Pressure
In a randomized, double-blind, parallel group, active control trial, Sumatriptan and Naproxen Sodium Tablets 85/500 mg administered intermittently over 6 months did not increase blood pressure in a normotensive adult population (n = 122). However, significant elevation in blood pressure has been reported with 5-HT1 agonists and NSAIDs in patients with and without a history of hypertension.
Close12.3 Pharmacokinetics
Absorption and Bioavailability
Sumatriptan, when given as Sumatriptan and Naproxen Sodium Tablets 85/500 mg, has a mean Cmax similar to that of sumatriptan succinate 100 mg tablets alone. The median Tmax of sumatriptan, when given as Sumatriptan and Naproxen Sodium Tablets 85/500 mg, was 1 hour (range: 0.3 to 4.0 hours), which is slightly different compared with sumatriptan succinate 100 mg tablets (median Tmax of 1.5 hours). Naproxen, when given as Sumatriptan and Naproxen Sodium Tablets 85/500 mg, has a Cmax which is approximately 36% lower than naproxen sodium 550 mg tablets and a median Tmax of 5 hours (range: 0.3 to 12 hours), which is approximately 4 hours later than from naproxen sodium tablets 550 mg. AUC values for sumatriptan and for naproxen are similar for Sumatriptan and Naproxen Sodium Tablets 85/500 mg compared with sumatriptan succinate 100 mg tablets or naproxen sodium 550 mg tablets, respectively. In a crossover trial in 16 subjects, the pharmacokinetics of both components administered as Sumatriptan and Naproxen Sodium Tablets 85/500 mg were similar during a migraine attack and during a migraine-free period.
Bioavailability of sumatriptan is approximately 15%, primarily due to presystemic (first-pass) metabolism and partly due to incomplete absorption.
Naproxen is absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%.
Food had no significant effect on the bioavailability of sumatriptan or naproxen administered as Sumatriptan and Naproxen Sodium Tablets, but slightly delayed the Tmax of sumatriptan by about 0.6 hour [see Dosage and Administration (2.3)].
Distribution
Plasma protein binding is 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The volume of distribution of sumatriptan is 2.7 L/kg.
The volume of distribution of naproxen is 0.16 L/kg. At therapeutic levels naproxen is greater than 99% albumin bound. At doses of naproxen greater than 500 mg/day, there is a less-than-proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough Css = 36.5, 49.2, and 56.4 mg/L with 500-; 1,000-; and 1,500-mg daily doses of naproxen, respectively). However, the concentration of unbound naproxen continues to increase proportionally to dose.
Metabolism
In vitro studies with human microsomes suggest that sumatriptan is metabolized by monoamine oxidase (MAO), predominantly the A isoenzyme. No significant effect was seen with an MAO-B inhibitor.
Naproxen is extensively metabolized to 6-0-desmethyl naproxen, and both parent and metabolites do not induce metabolizing enzymes.
Elimination
The elimination half-life of sumatriptan is approximately 2 hours. Radiolabeled 14C-sumatriptan administered orally is largely renally excreted (about 60%), with about 40% found in the feces. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive. Three percent of the dose can be recovered as unchanged sumatriptan.
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (less than 1%), 6-0-desmethyl naproxen (less than 1%), or their conjugates (66% to 92%). The plasma half-life of the naproxen anion in humans is approximately 19 hours. The corresponding half-lives of both metabolites and conjugates of naproxen are shorter than 12 hours, and their rates of excretion have been found to coincide closely with the rate of naproxen disappearance from the plasma. In patients with renal failure, metabolites may accumulate.
Specific Populations
Geriatrics
The pharmacokinetics of Sumatriptan and Naproxen Sodium Tablets in geriatric patients have not been studied. Elderly patients are more likely to have decreased hepatic function and decreased renal function [see Specific Populations (8.5)].
The pharmacokinetics of oral sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in patients with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years).
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction, which represents <1% of the total concentration, increased in the elderly (range of unbound trough naproxen from 0.12% to 0.19% in elderly subjects versus 0.05% to 0.075% in younger subjects).
Pediatrics
A pharmacokinetic study compared 3 doses of Sumatriptan and Naproxen Sodium Tablets in pediatric patients 12 to 17 years of age (n=24) with adults (n=26). The AUC and Cmax of sumatriptan were 50-60% higher following a single dose of Sumatriptan and Naproxen Sodium Tablets 10/60 mg in pediatric patients 12 to 17 years of age (n=7) compared with adult subjects (n=8), and were 6-26% higher following a single dose of Sumatriptan and Naproxen Sodium Tablets 30/180 mg or 85/500 mg in pediatrics than adults. Naproxen pharmacokinetic parameters were similar between pediatrics and adults.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of Sumatriptan and Naproxen Sodium Tablets has not been studied. Since naproxen and its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen metabolites to accumulate in the presence of renal insufficiency. Elimination of naproxen is decreased in patients with severe renal impairment [see Warnings and Precautions (5.12), Use in Specific Populations (8.6)].
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of Sumatriptan and Naproxen Sodium Tablets has not been studied. In a study in patients with moderate hepatic impairment (n = 8) matched for sex, age, and weight with healthy subjects (n = 8), patients with hepatic impairment had an approximately 70% increase in AUC and Cmax of sumatriptan and a Tmax 40 minutes earlier compared to healthy subjects. The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied.
Drug Interaction Studies
Aspirin
When naproxen was administered with aspirin (>1 gram/day), the protein binding of naproxen was reduced, although the clearance of free naproxen was not altered. See Table 3 for clinically significant drug interactions of naproxen, an NSAID, with aspirin [see Drug Interactions (7)].
Propranolol
Propranolol 80 mg given twice daily had no significant effect on sumatriptan pharmacokinetics. See Table 3 for clinically significant drug interactions of propranolol, a beta-blocker, with Sumatriptan and Naproxen Sodium Tablets [see Drug Interactions (7)].
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of Sumatriptan and Naproxen Sodium Tablets has not been studied. In carcinogenicity ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of Sumatriptan and Naproxen Sodium Tablets has not been studied.
In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 and 104 weeks, respectively, at doses up to 160 mg/kg/day. The highest doses tested are approximately 5 (mouse) and 9 (rat) times the maximum human daily dose (MHDD) of 170 mg sumatriptan on a mg/m2 basis (two tablets of Sumatriptan and Naproxen Sodium Tablets 85/500 mg in a 24-hour period).
The carcinogenic potential of naproxen was evaluated in a 2-year oral carcinogenicity study in rats at doses of 8, 16, and 24 mg/kg/day and in another 2-year oral carcinogenicity study in rats at a dose of 8 mg/kg/day. No evidence of tumorigenicity was found in either study. The highest dose tested is less than the MHDD (1000 mg) of naproxen, on a mg/m2 basis.
Mutagenesis
Sumatriptan and naproxen sodium tested alone and in combination were negative in an in vitro bacterial reverse mutation assay, and in an in vivo micronucleus assay in mice.
The combination of sumatriptan and naproxen sodium was negative in an in vitro mouse lymphoma tk assay in the presence and absence of metabolic activation. However, in separate in vitro mouse lymphoma tk assays, naproxen sodium alone was reproducibly positive in the presence of metabolic activation.
Naproxen sodium alone and in combination with sumatriptan was positive in an in vitro clastogenicity assay in mammalian cells in the presence and absence of metabolic activation. The clastogenic effect for the combination was reproducible within this assay and was greater than observed with naproxen sodium alone. Sumatriptan alone was negative in these assays.
Chromosomal aberrations were not induced in peripheral blood lymphocytes following 7 days of twice-daily dosing with Sumatriptan and Naproxen Sodium Tablets in human volunteers.
In previous studies, sumatriptan alone was negative in in vitro (bacterial reverse mutation [Ames], gene cell mutation in Chinese hamster V79/HGPRT, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility
The effect of Sumatriptan and Naproxen Sodium Tablets on fertility in animals has not been studied.
When sumatriptan (5, 50, 500 mg/kg/day) was administered orally to male and female rats prior to and throughout the mating period, there was a drug-related decrease in fertility secondary to a decrease in mating in animals treated with doses greater than 5 mg/kg/day (less than the MHDD of 170 mg on a mg/m2 basis). It is not clear whether this finding was due to an effect on males or females or both.
Close13.2 Animal Toxicology and/or Pharmacology
Corneal Opacities
Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dosage tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60-week study. Earlier examinations for these toxicities were not conducted and no-effect doses were not established. The lowest dose tested is less than the MHDD (170 mg) of sumatriptan on a mg/m2 basis.
-
14 CLINICAL STUDIES14.1 Adults - The efficacy of Sumatriptan and Naproxen Sodium Tablets in the acute treatment of migraine with or without aura in adults was demonstrated in 2 randomized, double-blind ...
14.1 Adults
The efficacy of Sumatriptan and Naproxen Sodium Tablets in the acute treatment of migraine with or without aura in adults was demonstrated in 2 randomized, double-blind, multicenter, parallel-group trials utilizing placebo and each individual active component of Sumatriptan and Naproxen Sodium Tablets 85/500 mg (sumatriptan and naproxen sodium) as comparison treatments (Study 1 and Study 2). Patients enrolled in these 2 trials were predominately female (87%) and white (88%), with a mean age of 40 years (range: 18 to 65 years). Patients were instructed to treat a migraine of moderate to severe pain with 1 tablet. No rescue medication was allowed within 2 hours postdose. Patients evaluated their headache pain 2 hours after taking 1 dose of study medication; headache relief was defined as a reduction in headache severity from moderate or severe pain to mild or no pain. Associated symptoms of nausea, photophobia, and phonophobia were also evaluated. Sustained pain free was defined as a reduction in headache severity from moderate or severe pain to no pain at 2 hours postdose without a return of mild, moderate, or severe pain and no use of rescue medication for 24 hours postdose. The results from Study 1 and 2 are summarized in Table 4. In both trials, the percentage of patients achieving headache pain relief 2 hours after treatment was significantly greater among patients receiving Sumatriptan and Naproxen Sodium Tablets 85/500 mg (65% and 57%) compared with those who received placebo (28% and 29%).
Further, the percentage of patients who remained pain free without use of other medications through 24 hours postdose was significantly greater among patients receiving a single dose of Sumatriptan and Naproxen Sodium Tablets 85/500 mg (25% and 23%) compared with those who received placebo (8% and 7%) or either sumatriptan (16% and 14%) or naproxen sodium (10%) alone.
Table 4. Percentage of Adult Patients with 2-Hour Pain Relief and Sustained Pain Free Following Treatment* Sumatriptan and Naproxen Sodium Tablets
85/500 mgSumatriptan
85 mgNaproxen Sodium
500 mgPlacebo 2-Hour Pain Relief Study 1 65%†
n = 36455%
n = 36144%
n = 35628%
n = 360Study 2 57%†
n = 36250%
n = 36243%
n = 36429%
n = 382Sustained Pain Free (2-24 Hours) Study 1 25%‡
n = 36416%
n = 36110%
n = 3568%
n = 360Study 2 23%‡
n = 36214%
n = 36210%
n = 3647%
n = 382The percentage of patients achieving initial headache pain relief within 2 hours following treatment with Sumatriptan and Naproxen Sodium Tablets 85/500 mg is shown in Figure 1.
Figure 1. Percentage of Adult Patients with Initial Headache Pain Relief within 2 Hours
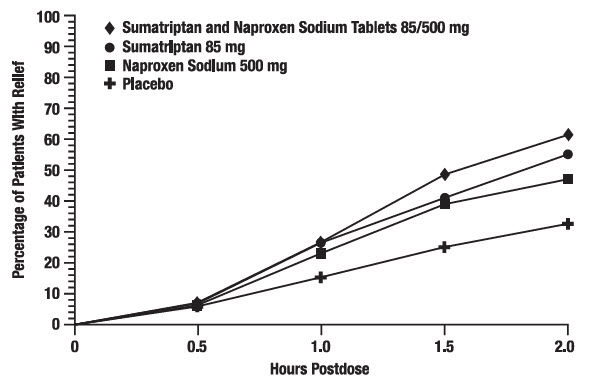
Compared with placebo, there was a decreased incidence of photophobia, phonophobia, and nausea 2 hours after the administration of Sumatriptan and Naproxen Sodium Tablets 85/500 mg. The estimated probability of taking a rescue medication over the first 24 hours is shown in Figure 2.
Figure 2. Estimated Probability of Adults Taking a Rescue Medication over the 24 Hours following the First Dose1
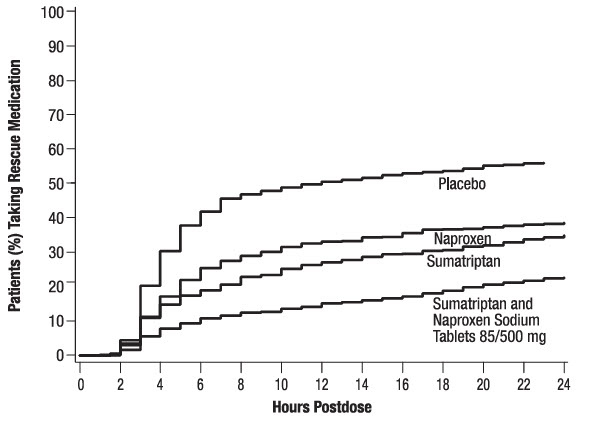
Sumatriptan and Naproxen Sodium Tablets 85/500 mg was more effective than placebo regardless of the presence of aura; duration of headache prior to treatment; gender, age, or weight of the subject; or concomitant use of oral contraceptives or common migraine prophylactic drugs (e.g., beta-blockers, anti-epileptic drugs, tricyclic antidepressants).
- 1
- Kaplan-Meier plot based on data obtained in the 2 clinical controlled trials providing evidence of efficacy with patients not using additional treatments censored to 24 hours. Plot also includes patients who had no response to the initial dose. No rescue medication was allowed within 2 hours postdose.
Close14.2 Pediatric Patients 12 to 17 Years of Age
The efficacy of Sumatriptan and Naproxen Sodium Tablets in the acute treatment of migraine with or without aura in pediatric patients 12 to 17 years of age was demonstrated in a randomized, double-blind, multicenter, parallel-group, placebo-controlled, multicenter trial comparing 3 doses of Sumatriptan and Naproxen Sodium Tablets and placebo (Study 3). Patients enrolled in this trial were mostly female (59%) and white (81%), with a mean age of 15 years.
Patients were required to have at least a 6-month history of migraine attacks with or without aura usually lasting 3 hours or more when untreated. Following a single-blind, placebo run-in phase, placebo nonresponders were randomized to receive a single dose of either Sumatriptan and Naproxen Sodium Tablets 10/60 mg, 30/180 mg, 85/500 mg, or placebo. Patients were instructed to treat a single migraine attack with headache pain of moderate to severe intensity. No rescue medication was allowed within 2 hours postdose. Patients evaluated their headache pain 2 hours after taking 1 dose of study medication. Two-hour pain free was defined as a reduction in headache severity from moderate or severe pain to no pain at 2 hours postdose.
Results are summarized in Table 5. The percentage of patients who were pain free at 2 hours postdose was significantly greater among patients who received any of the 3 doses of Sumatriptan and Naproxen Sodium Tablets compared with placebo.
Table 5. Percentage of Pediatric Patients 12 to 17 Years of Age with 2-Hour Pain-Free Response Following Treatment in Study 3* Endpoint Sumatriptan and Naproxen Sodium Tablets 10/60 mg
(n = 96)Sumatriptan and Naproxen Sodium Tablets 30/180 mg
(n = 97)Sumatriptan and Naproxen Sodium Tablets 85/500 mg
(n = 152)Placebo
(n = 145)2-Hour Pain Free 29%† 27%† 24%† 10% The percentage of pediatric patients who remained pain free without use of other medications 2 through 24 hours postdose was significantly greater after administration of a single dose of Sumatriptan and Naproxen Sodium Tablets 85/500 mg compared with placebo. A greater percentage of pediatric patients who received a single dose of 10/60 mg or 30/180 mg remained pain free 2 through 24 hours postdose compared with placebo.
Compared with placebo, the incidence of photophobia and phonophobia was significantly decreased 2 hours after the administration of a single dose of 85/500 mg, whereas, the incidence of nausea was comparable. There was a decreased incidence of photophobia, phonophobia, and nausea 2 hours after single-dose administration of 10/60 mg or 30/180 mg compared with placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSumatriptan and Naproxen Sodium Tablets 85/500 mg contains 119 mg of sumatriptan succinate equivalent to 85 mg of sumatriptan and 500 mg of naproxen sodium and is supplied as blue film-coated ...
Sumatriptan and Naproxen Sodium Tablets 85/500 mg contains 119 mg of sumatriptan succinate equivalent to 85 mg of sumatriptan and 500 mg of naproxen sodium and is supplied as blue film-coated tablets debossed on one side with TREXIMET in bottles of 9 tablets with desiccant (NDC 44183-850-09).
Sumatriptan and Naproxen Sodium Tablets 10/60 mg contains 14 mg of sumatriptan succinate equivalent to 10 mg of sumatriptan and 60 mg of naproxen sodium and is supplied as light-blue film-coated tablets debossed on one side with TREXIMET and the other side with 10-60 in bottles of 9 tablets with desiccant (NDC 44183-860-09).
CloseStore at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Do not repackage; dispense and store in original container with desiccant.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with Sumatriptan and Naproxen Sodium Tablets and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events, Prinzmetal's Angina, Other Vasospasm-Related Events, Arrhythmias and Cerebrovascular Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic effects such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for signs and symptoms of chest pain, shortness of breath, weakness, irregular heartbeat, significant rise in blood pressure, weakness and slurring of speech, and should be advised to report any of these symptoms to their health care provider immediately. Apprise patients of the importance of this follow-up [see Warnings and Precautions (5.1, 5.3, 5.5, 5.6, 5.8)].
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [see Warnings and Precautions (5.2)].
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, instruct patients to stop Sumatriptan and Naproxen Sodium Tablets and seek immediate medical therapy [see Warnings and Precautions (5.7)].
Anaphylactic Reactions
Inform patients that anaphylactic reactions have occurred in patients receiving the components of Sumatriptan and Naproxen Sodium Tablets. Such reactions can be life-threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help [see Contraindications (4), Warnings and Precautions (5.13)].
Serious Skin Reactions, including DRESS
Inform patients that Sumatriptan and Naproxen Sodium Tablets, like other NSAID-containing products, may increase the risk of serious skin side effects such as exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching and should ask for medical advice when observing any indicative signs or symptoms. Advise patients to stop taking Sumatriptan and Naproxen Sodium Tablets immediately if they develop any type of rash or fever and contact their healthcare providers as soon as possible [see Warnings and Precautions (5.14, 5.15)].
Fetal Toxicity
Inform pregnant women to avoid use of Sumatriptan and Naproxen Sodium Tablets and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with Sumatriptan and Naproxen Sodium Tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [see Warnings and Precautions (5.16), Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, may be associated with a reversible delay in ovulation [see Use in Specific Populations (8.3)].
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see Warnings and Precautions (5.9)].
Concomitant Use with Other Triptans or Ergot Medications
Inform patients that use of Sumatriptan and Naproxen Sodium Tablets within 24 hours of another triptan or an ergot-type medication (including dihydroergotamine or methysergide) is contraindicated [see Contraindications (4), Drug Interactions (7.1)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with the use of Sumatriptan and Naproxen Sodium Tablets or other triptans, particularly during concomitant use with SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.11), Drug Interactions (7.1)].
Medication Overuse Headache
Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.10)].
Ability to Perform Complex Tasks
Treatment with Sumatriptan and Naproxen Sodium Tablets may cause somnolence and dizziness; instruct patients to evaluate their ability to perform complex tasks after administration of Sumatriptan and Naproxen Sodium Tablets [see Adverse Reactions (6.1)].
Asthma
Advise patients with preexisting asthma to seek immediate medical attention if their asthma worsens after taking Sumatriptan and Naproxen Sodium Tablets. Patients with a history of aspirin-sensitive asthma should not take Sumatriptan and Naproxen Sodium Tablets [see Contraindications (4), Warnings and Precautions (5.18)].
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of Sumatriptan and Naproxen Sodium Tablets with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [see Warnings and Precautions (5.2) and Drug Interactions (7)]. Alert patients that NSAIDs may be present in "over the counter" medications for treatment of colds, fever, or insomnia.
CloseUse of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with Sumatriptan and Naproxen Sodium Tablets until they talk to their healthcare provider [see Drug Interactions (7)].
-
SPL UNCLASSIFIED SECTIONThe other brands listed are trademarks of their respective owners and are not trademarks of Currax™ Pharmaceuticals LLC. The makers of these brands are not affiliated with and do not endorse ...
The other brands listed are trademarks of their respective owners and are not trademarks of Currax™ Pharmaceuticals LLC. The makers of these brands are not affiliated with and do not endorse Currax™ Pharmaceuticals LLC or its products.
Distributed by Macoven
Brentwood, TN 37027© 2024 Currax™ Pharmaceuticals LLC. All rights reserved.
SUM-LC-089.05
Close -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 02/2024 MEDICATION GUIDE - Sumatriptan and Naproxen Sodium Tablets - Read this Medication ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 02/2024 MEDICATION GUIDE
Sumatriptan and Naproxen Sodium TabletsRead this Medication Guide before you start taking Sumatriptan and Naproxen Sodium Tablets and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment. What is the most important information I should know about Sumatriptan and Naproxen Sodium Tablets?
Sumatriptan and Naproxen Sodium Tablets may increase your chance of a heart attack or stroke that can lead to death. Sumatriptan and Naproxen Sodium Tablets contain 2 medicines: sumatriptan and naproxen sodium (a nonsteroidal anti-inflammatory drug [NSAID]).- This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
Do not take Sumatriptan and Naproxen Sodium Tablets right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
Avoid taking Sumatriptan and Naproxen Sodium Tablets after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
Stop taking Sumatriptan and Naproxen Sodium Tablets and get emergency help right away if you have any of the following symptoms of a heart attack or stroke:- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- weakness in one part or on one side of your body
- slurred speech
Sumatriptan and Naproxen Sodium Tablets is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you: - have high blood pressure
- smoke
- have diabetes
- have high cholesterol levels
- are overweight
- have a family history of heart disease
Sumatriptan and Naproxen Sodium Tablets can cause ulcers and bleeding in the stomach and intestines at any time during your treatment.
Ulcers and bleeding can happen without warning symptoms and may cause death.
Your chance of getting an ulcer or bleeding increases with:- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- the use of medicines called "corticosteroids," "anticoagulants," and antidepressant medicines called "SSRIs" or "SNRIs"
- more frequent use
- drinking alcohol
- having poor health
- advanced liver disease
- bleeding problems
- longer use
- smoking
- older age
Sumatriptan and Naproxen Sodium Tablets may cause serious allergic reactions or serious skin reactions that can be life-threatening. Stop taking Sumatriptan and Naproxen Sodium Tablets and get emergency help right away if you develop: - sudden wheezing
- rash
- problems breathing or swallowing
- blisters or bleeding of your lips, eye lids, mouth, nose, or genitals
- swelling of your lips, tongue, throat or body
- fainting
- reddening of your skin with blisters or peeling
Sumatriptan and Naproxen Sodium Tablets should only be used exactly as prescribed, at the lowest dose possible for your treatment, and for the shortest time needed.
Sumatriptan and Naproxen Sodium Tablets already contains an NSAID (naproxen). Do not use Sumatriptan and Naproxen Sodium Tablets with other medicines to lessen pain or fever or with other medicines for colds or sleeping problems without talking to your healthcare provider first, because they may contain an NSAID also.What is Sumatriptan and Naproxen Sodium Tablets?
Sumatriptan and Naproxen Sodium Tablets is a prescription medicine that contains sumatriptan and naproxen sodium (an NSAID). Sumatriptan and Naproxen Sodium Tablets is used to treat acute migraine headaches with or without aura in patients 12 years of age and older.
Sumatriptan and Naproxen Sodium Tablets is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
Sumatriptan and Naproxen Sodium Tablets is not used to prevent or decrease the number of migraine headaches you have.
It is not known if Sumatriptan and Naproxen Sodium Tablets is safe and effective to treat cluster headaches.Who should not take Sumatriptan and Naproxen Sodium Tablets?
Do not take Sumatriptan and Naproxen Sodium Tablets if you have:- heart problems, history of heart problems, or right before or after heart bypass surgery
- had a stroke, transient ischemic attack (TIAs), or problems with your blood circulation
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- narrowing of blood vessels to your legs and arms (peripheral vascular disease), stomach (ischemic bowel disease), or kidneys
- uncontrolled high blood pressure
- taken any medicines in the last 24 hours that are called 5-HT1 agonists that are triptans or contain ergotamine. Ask your healthcare provider for a list of these medicines if you are not sure.
- taken an antidepressant medicine called a monoamine oxidase (MAO) inhibitor within the last 2 weeks. Ask your healthcare provider for a list if you are not sure.
- had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- an allergy to sumatriptan, naproxen, or any of the ingredients in Sumatriptan and Naproxen Sodium Tablets. See "What are the ingredients in Sumatriptan and Naproxen Sodium Tablets?" below for a complete list of ingredients.
- liver problems
What should I tell my healthcare provider before taking Sumatriptan and Naproxen Sodium Tablets?
Before you take Sumatriptan and Naproxen Sodium Tablets, tell your healthcare provider about all of your medical conditions, including if you:- have high blood pressure
- have asthma
- have high cholesterol
- have diabetes
- smoke
- are overweight
- have heart problems or a family history of heart problems or stroke
- have kidney problems
- have liver problems
- have had epilepsy or seizures
- are not using effective birth control
- are pregnant, think you might be pregnant, or are trying to become pregnant. Taking NSAIDs, including Sumatriptan and Naproxen Sodium Tablets, at about 20 weeks of pregnancy or later may harm your unborn baby. If you need to take NSAIDs for more than 2 days when you are between 20 and 30 weeks of pregnancy, your healthcare provider may need to monitor the amount of fluid in your womb around your baby. You should not take NSAIDs after about 30 weeks of pregnancy.
- are breastfeeding or plan to breastfeed. The components of Sumatriptan and Naproxen Sodium Tablets pass into your breast milk and may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take Sumatriptan and Naproxen Sodium Tablets.
Sumatriptan and Naproxen Sodium Tablets and certain other medicines can affect each other, causing serious side effects.How should I take Sumatriptan and Naproxen Sodium Tablets? - Certain people should take their first dose of Sumatriptan and Naproxen Sodium Tablets in their healthcare provider's office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Take Sumatriptan and Naproxen Sodium Tablets exactly as your healthcare provider tells you to take it.
- Take Sumatriptan and Naproxen Sodium Tablets whole with water or other liquids.
- Sumatriptan and Naproxen Sodium Tablets can be taken with or without food.
- If you do not get any relief after your first dose, do not take a second dose without first talking with your healthcare provider.
- If your headache comes back or you only get some relief from your headache:
- For adults: a second dose may be taken 2 hours after the first dose. Do not take more than 2 doses of Sumatriptan and Naproxen Sodium Tablets 85/500 mg in a 24-hour period.
- For children 12 to 17 years of age: it is not known if taking more than 1 dose of Sumatriptan and Naproxen Sodium Tablets in 24 hours is safe and effective. Talk to your healthcare provider about what to do if your headache does not go away or comes back.
- If you take too much Sumatriptan and Naproxen Sodium Tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take Sumatriptan and Naproxen Sodium Tablets so you can talk with your healthcare provider about how Sumatriptan and Naproxen Sodium Tablets is working for you.
What should I avoid while taking Sumatriptan and Naproxen Sodium Tablets?
Sumatriptan and Naproxen Sodium Tablets can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.What are the possible side effects of Sumatriptan and Naproxen Sodium Tablets?
Sumatriptan and Naproxen Sodium Tablets may cause serious side effects. See "What is the most important information I should know about Sumatriptan and Naproxen Sodium Tablets?"
These serious side effects include:- changes in color or sensation in your fingers and toes (Raynaud's syndrome)
- new or worse high blood pressure
- heart failure from body swelling (fluid retention)
- kidney problems including kidney failure
- low red blood cells (anemia)
- liver problems including liver failure
- asthma attacks in people who have asthma
- stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- weight loss
- constipation or diarrhea
- fever
- stomach pain after meals
- nausea or vomiting
- bloody diarrhea
- problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- medication overuse headaches. Some people who use too many Sumatriptan and Naproxen Sodium Tablets may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with Sumatriptan and Naproxen Sodium Tablets.
- serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using Sumatriptan and Naproxen Sodium Tablets, especially if Sumatriptan and Naproxen Sodium Tablets is used with antidepressant medicines called SSRIs or SNRIs. Stop taking Sumatriptan and Naproxen Sodium Tablets and call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
- changes in blood pressure
- tight muscles
- mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- fast heartbeat
- high body temperature
- trouble walking
- seizures. Seizures have happened in people taking sumatriptan, one of the ingredients in Sumatriptan and Naproxen Sodium Tablets, who have never had seizures before. Talk with your healthcare provider about your chance of having seizures while you take Sumatriptan and Naproxen Sodium Tablets.
The most common side effects of Sumatriptan and Naproxen Sodium Tablets include: - dizziness
- pain, discomfort, or stiffness in your neck, throat, jaw, or chest
- tingling or numbness in your fingers or toes
- dry mouth
- heartbeat problems
- feeling weak, drowsy, or tired
- nausea
- heartburn
- feeling hot
- muscle tightness
Stop Sumatriptan and Naproxen Sodium Tablets and call your healthcare provider right away if you have any of the following symptoms: - nausea that seems out of proportion to your migraine
- vomit blood
- yellow skin or eyes
- more tired or weaker than usual
- itching
- swelling of the arms, legs, hands, and feet
- sudden or severe stomach pain
- blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- flu-like symptoms
- diarrhea
- tenderness in your upper right side
Tell your healthcare provider if you have any side effects that bother you or do not go away.
These are not all of the side effects of Sumatriptan and Naproxen Sodium Tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Sumatriptan and Naproxen Sodium Tablets?
Store Sumatriptan and Naproxen Sodium Tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Sumatriptan and Naproxen Sodium Tablets and all medicines out of the reach of children.General information about the safe and effective use of Sumatriptan and Naproxen Sodium Tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Sumatriptan and Naproxen Sodium Tablets for a condition for which it was not prescribed. Do not give Sumatriptan and Naproxen Sodium Tablets to other people, even if they have the same problem you have. It may harm them.
This Medication Guide summarizes the most important information about Sumatriptan and Naproxen Sodium Tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Sumatriptan and Naproxen Sodium Tablets that is written for healthcare professionals.
For more information call 1-800-793-2145.What are the ingredients in Sumatriptan and Naproxen Sodium Tablets?
Active ingredients: sumatriptan succinate and naproxen sodium.
Inactive ingredients in all strengths: croscarmellose sodium, dibasic calcium phosphate, FD&C Blue No. 2, magnesium stearate, microcrystalline cellulose, povidone, sodium bicarbonate, talc, and titanium dioxide.
85/500-mg tablets also contain: hypromellose and triacetin.
10/60-mg tablets also contain: polyethylene glycol and polyvinyl alcohol.
The other brands listed are trademarks of their respective owners and are not trademarks of Currax™ Pharmaceuticals LLC. The makers of these brands are not affiliated with and do not endorse Currax™ Pharmaceuticals LLC or its products.
SUM-LC090.04
Distributed by Macoven;
Brentwood, TN 37027 © 2024 Currax™ Pharmaceuticals LLC.
All rights reserved. - This risk may happen early in treatment and may increase:
-
PRINCIPAL DISPLAY PANEL - 9 Tablet Bottle LabelNDC 44183-850-09 - Sumatriptan and - Naproxen Sodium Tablets - 85 mg/500 mg - Do not repackage; dispense and store - in original container. Dispense the accompanying - Medication Guide to each patient. Rx ...
NDC 44183-850-09
Sumatriptan and
Naproxen Sodium Tablets85 mg/500 mg
Do not repackage; dispense and store
in original container.Dispense the accompanying
Medication Guide to each patient.Rx Only
9 TABLETSMACOVEN
Close
-
INGREDIENTS AND APPEARANCEProduct Information
SUMATRIPTAN SUCCINATE AND NAPROXEN SODIUM sumatriptan succinate and naproxen sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44183-850 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sumatriptan Succinate (UNII: J8BDZ68989) (Sumatriptan - UNII:8R78F6L9VO) Sumatriptan 85 mg Naproxen Sodium (UNII: 9TN87S3A3C) (Naproxen - UNII:57Y76R9ATQ) Naproxen Sodium 500 mg Inactive Ingredients Ingredient Name Strength Croscarmellose Sodium (UNII: M28OL1HH48) Anhydrous Dibasic Calcium Phosphate (UNII: L11K75P92J) FD&C Blue No. 2 (UNII: L06K8R7DQK) Magnesium Stearate (UNII: 70097M6I30) Microcrystalline Cellulose (UNII: OP1R32D61U) Povidone, Unspecified (UNII: FZ989GH94E) Sodium Bicarbonate (UNII: 8MDF5V39QO) Talc (UNII: 7SEV7J4R1U) Titanium Dioxide (UNII: 15FIX9V2JP) Hypromellose, Unspecified (UNII: 3NXW29V3WO) Triacetin (UNII: XHX3C3X673) Product Characteristics Color BLUE Score no score Shape OVAL Size 19mm Flavor Imprint Code TREXIMET Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44183-850-09 9 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA021926 05/14/2015 Labeler - Currax Pharmaceuticals LLC dba Cypress, Hawthorn, Macoven (117055730) Establishment Name Address ID/FEI Business Operations Cambrex 829609168 ANALYSIS(44183-850) , MANUFACTURE(44183-850) , PACK(44183-850) Establishment Name Address ID/FEI Business Operations Industrias Quimicas Falcon de Mexico, S.A. de C.V. (Dr. Reddy's Mexico) 812915445 API MANUFACTURE(44183-850)
CloseEstablishment Name Address ID/FEI Business Operations Dr. Reddy's Laboratories Limited (India) 918607727 API MANUFACTURE(44183-850)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
SUMATRIPTAN SUCCINATE AND NAPROXEN SODIUM tablet
Get Label RSS Feed for this Drug
SUMATRIPTAN SUCCINATE AND NAPROXEN SODIUM tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=5762bbe6-bddf-495c-adf8-9096f98a3a9c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
SUMATRIPTAN SUCCINATE AND NAPROXEN SODIUM tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 44183-850-09 |

