Label: BEXAROTENE capsule, liquid filled
- NDC Code(s): 43975-315-10
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BEXAROTENE CAPSULES safely and effectively. See full prescribing information for BEXAROTENE CAPSULES. BEXAROTENE capsules, for ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE Bexarotene capsules are indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy.
-
2 DOSAGE AND ADMINISTRATION The recommended initial dose of bexarotene capsules is 300 mg/m2/day (see Table 1). Bexarotene capsules should be taken as a single oral daily dose with a meal. For precautions to prevent ...
-
3 DOSAGE FORMS AND STRENGTHS Capsules: 75 mg, off-white, oblong soft gelatin capsules, imprinted “A78” with black ink.
-
4 CONTRAINDICATIONS 4.1 Pregnancy - Bexarotene can cause fetal harm when administered to a pregnant female. Bexarotene is a member of the retinoid class of drugs that is associated with birth defects in humans and ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hyperlipidemia - Bexarotene induces substantial elevations in lipids in most patients. About 70% of patients with CTCL who received an initial dose of ≥300 mg/m2/day of bexarotene capsules ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information: • Hyperlipidemia [see Warnings and Precautions (5.1)] • Pancreatitis [see ...
-
7 DRUG INTERACTIONS Effect of Other Drugs on Bexarotene Capsules - Gemfibrozil: Concomitant administration of bexarotene capsules and gemfibrozil resulted in increases in plasma concentrations of bexarotene ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Bexarotene, a retinoid, can cause fetal harm based on findings from animal studies when administered to a pregnant female and is contraindicated during pregnancy ...
-
10 OVERDOSAGE Doses up to 1000 mg/m2/day of bexarotene capsules have been administered in short-term trials in patients with advanced cancer without acute toxic effects. Single doses of 1500 mg/kg and 720 mg/kg ...
-
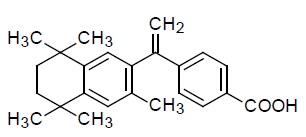
11 DESCRIPTION Bexarotene capsules contain bexarotene, a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Bexarotene selectively binds and activates retinoid X receptor subtypes (RXRα, RXRß, RXRγ). RXRs can form heterodimers with various receptor partners such as retinoic ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to assess the carcinogenic potential of bexarotene have not been conducted. Bexarotene is not mutagenic to ...
-
14 CLINICAL STUDIES Bexarotene capsules were evaluated in two clinical trials in 152 patients with advanced and early stage cutaneous T-cell lymphoma (CTCL) in two multicenter, open-label, historically-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Bexarotene capsules are supplied as 75 mg off-white, opaque, oblong soft gelatin capsules, imprinted with black ink “A78”, in high density polyethylene bottles with child-resistant ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Inform the patient or caregiver about the following: Birth Defects - Advise patients that bexarotene is ...
-
Patient Information Bexarotene Capsules (beks-AIR-o-teen capsules) What is the most important information I should know about bexarotene capsules? Bexarotene capsules can cause serious side effects, including ...
-
Package/Label Display Panel NDC 43975-315-10 - Bexarotene Capsules - 75 mg - Rx only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information