Label: CEFIXIME powder, for suspension
- NDC Code(s): 43598-673-50, 43598-674-50, 43598-674-51
- Packager: Dr. Reddy’s Laboratories, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CEFIXIME FOR ORAL SUSPENSION safely and effectively. See full prescribing information for CEFIXIME FOR ORAL SUSPENSION. CEFIXIME ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGETo reduce the development of drug resistant bacteria and maintain the effectiveness of Cefixime for oral suspension and other antibacterial drugs, Cefixime for oral suspension should be used only ...
-
2. DOSAGE AND ADMINISTRATION2.1 Adults - The recommended dose of cefixime is 400 mg daily. This may be given as a 400 mg tablet or capsule daily or the 400 mg tablet may be split and given as one half tablet every 12 hours ...
-
3 DOSAGE FORMS AND STRENGTHSCefixime for oral suspension,USP is available for oral administration in the following dosage forms and strengths: Powder for oral suspension, when reconstituted, provides either 100 mg/5 mL or ...
-
4 CONTRAINDICATIONSCefixime for oral suspension is contraindicated in patients with known allergy to cefixime or other cephalosporins.
-
5. WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Anaphylactic/anaphylactoid reactions (including shock and fatalities) have been reported with the use of cefixime. Before therapy with Cefixime for oral ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Carbamazepine - Elevated carbamazepine levels have been reported in postmarketing experience when cefixime is administered concomitantly. Drug monitoring may be of assistance in detecting ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category B. Reproduction studies have been performed in mice and rats at doses up to 40 times the human dose and have revealed no evidence of harm to the fetus due to ...
-
10 OVERDOSAGEGastric lavage may be indicated; otherwise, no specific antidote exists. Cefixime is not removed in significant quantities from the circulation by hemodialysis or peritoneal dialysis. Adverse ...
-
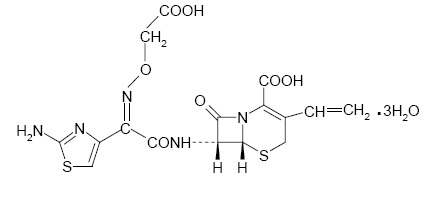
11 DESCRIPTIONCefixime is a semisynthetic, cephalosporin antibacterial for oral administration. Chemically, it is (6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo [4.2.0 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Cefixime is a semisynthetic cephalosporin antibacterial drug [ see Microbiology( 12.4)]. 12.3 Pharmacokinetics - Cefixime ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Lifetime studies in animals to evaluate carcinogenic potential have not been conducted. Cefixime did not cause point mutations in ...
-
14 CLINICAL STUDIESComparative clinical trials of otitis media were conducted in nearly 400 children between the ages of 6 months to 10 years. Streptococcuspneumoniae was isolated from 47% of the ...
-
15 REFERENCES1. Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Tenth Edition. CLSI document ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCefixime for oral suspension, USP is available for oral administration in following dosage forms, strengths and packages listed in the table below: Dosage Form Strength Description Package ...
-
17 PATIENT COUNSELING INFORMATION17.1 Information for Patients - Patients should be counseled that antibacterial drugs, including cefixime, should only be used to treat bacterial infections. They do not treat viral infections ...
-
PRINCIPAL DISPLAY PANELCEFIXIME FOR ORAL SUSPENSION, USP - 100 mg/5 mL - Rx only - NDC : 43598-673-50 Bottle of - 50 mL - CEFIXIME FOR ORAL SUSPENSION, USP - 200 mg/5 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information