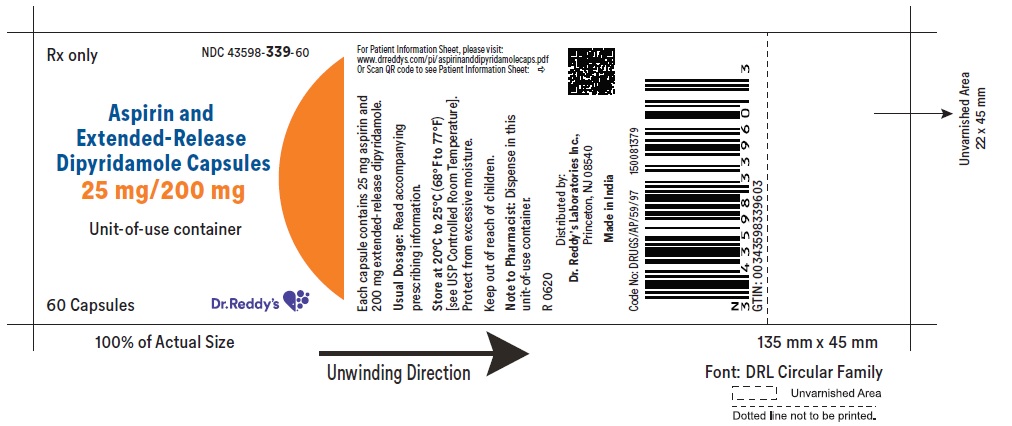
Aspirin and Extended-Release Dipyridamole Capsules
-
(as' pir in and dye'' pir id' a mole)
Read this Patient Information before you start taking aspirin and extended-release dipyridamole ...
Aspirin and Extended-Release Dipyridamole Capsules
(as' pir in and dye'' pir id' a mole)
Read this Patient Information before you start taking aspirin and extended-release dipyridamole capsules and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is aspirin and extended-release dipyridamole capsules?
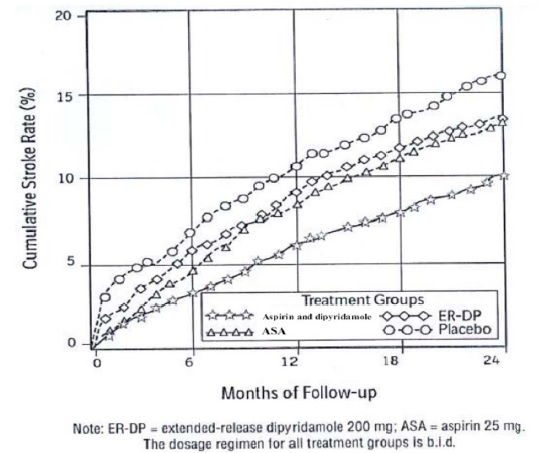
Aspirin and extended-release dipyridamole capsule is a prescription medicine that contains aspirin and a medicine that is slowly released in your body, called dipyridamole. Aspirin and extended-release dipyridamole capsules are used to lower the risk of stroke in people who have had a “mini-stroke” (transient ischemic attack or TIA) or stroke due to a blood clot.
It is not known if aspirin and extended-release dipyridamole capsules are safe and effective in children. See “Who should not take aspirin and extended-release dipyridamole capsules?”
Who should not take aspirin and extended-release dipyridamole capsules?
Do not take aspirin and extended-release dipyridamole capsules if you:
• are allergic to any of the ingredients in aspirin and extended-release dipyridamole capsules. See the end of this leaflet for a list of ingredients in aspirin and extended-release dipyridamole capsules.
• are allergic to non-steroidal anti-inflammatory drugs (NSAIDs)
• have asthma in combination with runny nose and nasal polyps
Do not give aspirin and extended-release dipyridamole capsules to a child or teenager with a viral illness. Reye syndrome, a life-threatening condition, can happen when aspirin (an ingredient in aspirin and extended-release dipyridamole capsules) is used in children and teenagers who have certain viral illnesses.
What should I tell my doctor before using aspirin and extended-release dipyridamole capsules?
Before taking aspirin and extended-release dipyridamole capsules, tell your healthcare provider if you:
• have stomach ulcers
• have a history of bleeding problems
• have heart problems
• have kidney or liver problems
• have low blood pressure
• have myasthenia gravis
• have any other medical conditions
• are pregnant or plan to become pregnant. You should not take aspirin and extended-release dipyridamole capsules during pregnancy without first talking to your healthcare provider. Tell your healthcare provider right away if you become pregnant while taking aspirin and extended-release dipyridamole capsules.
• are breast-feeding or plan to breast-feed. Aspirin and dipyridamole can pass into your milk. Talk to your healthcare provider about the best way to feed your baby if you take aspirin and extended-release dipyridamole capsules.
Tell your doctor you are taking aspirin and extended-release dipyridamole capsules if you are scheduled to have a stress test for your heart.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Aspirin and extended-release dipyridamole capsules and other medicines may affect each other causing side effects. Aspirin and extended-release dipyridamole capsules may affect the way other medicines work, and other medicines may affect how aspirin and extended-release dipyridamole capsules works. Especially tell your healthcare provider if you take:
• a medicine for high blood pressure, irregular heart beat, or heart failure
• acetazolamide [Diamox®]
• any blood thinner medicines
• warfarin sodium [Coumadin®, Jantoven®]
• a heparin medicine
• anagrelide [Agrylin®]
• a seizure medicine
• a medicine for Alzheimer’s disease
• a water pill
• methotrexate sodium [Trexall®]
• aspirin or a non-steroidal anti-inflammatory drug (NSAIDs). You should not take NSAIDs during treatment with aspirin and extended-release dipyridamole capsules. Using these medicines with aspirin and extended-release dipyridamole capsules can increase your risk of bleeding.
• a medicine for diabetes
• probenecid [Probalan®, Col-Probenecid®]
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show your healthcare provider and pharmacist when you get a new medicine.
How should I take aspirin and extended-release dipyridamole capsules?
• Take aspirin and extended-release dipyridamole capsules exactly as prescribed. Your healthcare provider will tell you how many aspirin and extended-release dipyridamole capsules to take and when to take them.
• Headaches are not uncommon when you first start taking aspirin and extended-release dipyridamole capsules, but often lessen as treatment continues. Tell your healthcare provider if you have a severe headache. Your healthcare provider may change the instructions for taking aspirin and extended-release dipyridamole capsules.
• Swallow aspirin and extended-release dipyridamole capsules whole. Do not crush or chew the capsules.
• You can take aspirin and extended-release dipyridamole capsules with or without food.
• If you miss a dose, take your next dose at the usual time. Do not take two doses at one time.
• If you take more aspirin and extended-release dipyridamole capsules (overdose) than prescribed, call your healthcare provider or Poison Control Center, or get emergency help right away.
Symptoms of an overdose of aspirin and extended-release dipyridamole capsules include:
• a warm feeling or flushing
• sweating
• restlessness
• weakness or dizziness
• a fast heart rate
• ringing in the ears
What should I avoid while using aspirin and extended-release dipyridamole capsules?
• heavy alcohol use. People who drink three or more alcoholic drinks every day have a higher risk of bleeding during treatment with aspirin and extended-release dipyridamole capsules, because it contains aspirin.
What are the possible side effects of aspirin and extended-release dipyridamole capsules?
Aspirin and extended-release dipyridamole capsules may cause serious side effects, including:
• increased risk of bleeding. You may bleed more easily during aspirin and extended-release dipyridamole capsules treatment, and it may take longer than usual for bleeding to stop. This can include:
• bleeding into your brain (intracranial hemorrhage). This can be a medical emergency. Get medical help right away if you have any of these symptoms while taking aspirin and extended-release dipyridamole capsules:
• severe headache with drowsiness
• confusion or memory change
• pass out (become unconscious)
• bleeding in your stomach or intestine.
• stomach pain
• heartburn or nausea
• vomiting blood or vomit looks like “coffee grounds”
• red or bloody stools
• black stools that look like tar
• new or worsening chest pain in some people with heart disease. Tell your healthcare provider if you have new chest pain or have any change in your chest pain during treatment with aspirin and extended-release dipyridamole capsules.
• liver problems, including increased liver function tests and liver failure. Tell your healthcare provider if you have any of these symptoms of a liver problem while taking aspirin and extended-release dipyridamole capsules:
• loss of appetite
• pale colored stool
• stomach area (abdomen) pain
• yellowing of your skin or whites of your eyes
• dark urine
• itching
Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of aspirin and extended-release dipyridamole capsules include:
• headache
• upset stomach
• diarrhea
These are not all the possible side effects of aspirin and extended-release dipyridamole capsules. Tell your healthcare provider or pharmacist if you have any side effect that bothers you or that does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA1088.
How should I store aspirin and extended-release dipyridamole capsules?
• Store aspirin and extended-release dipyridamole capsules at 20ºC to 25ºC (68ºF to 77ºF).
• Keep aspirin and extended-release dipyridamole capsules dry.
Keep aspirin and extended-release dipyridamole capsules and all medicines out of the reach of children.
General information about aspirin and extended-release dipyridamole capsules
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use aspirin and extended-release dipyridamole capsules for a condition for which it was not prescribed. Do not give aspirin and extended-release dipyridamole capsules to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about aspirin and extended-release dipyridamole capsules. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about aspirin and extended-release dipyridamole capsules that is written for health professionals.
For more information, call Dr. Reddy's Laboratories, Inc. at 1-888-375-3784
What are the ingredients in aspirin and extended-release dipyridamole capsules?
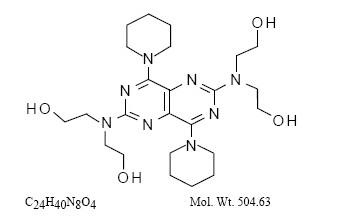
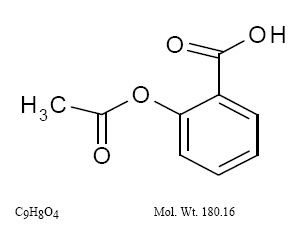
Active Ingredients: dipyridamole in an extended-release form and aspirin
Inactive Ingredients: acacia, anhydrous lactose, colloidal silicon dioxide, dimethicone, hypromellose, hypromellose phthalate, lecithin, methacrylic acid copolymer, microcrystalline cellulose, polyvinyl alcohol, povidone, pregelatinized starch, stearic acid, talc, tartaric acid, titanium dioxide, triacetin and xanthan gum.
Each capsule shell contains FD&C yellow 6, gelatin, sodium lauryl sulfate, titanium dioxide and yellow iron oxide.
Imprinting ink contains black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
The other brands listed are trademarks of their respective owners and are not trademarks of Dr. Reddy’s Laboratories Inc.
Rx only
Distributed by:
Dr. Reddy’s Laboratories Inc.,
Princeton, NJ 08540
Made in India
Revised: 0521
For Patient Information Sheet, please visit:
www.drreddys.com/pi/aspirinanddipyridamolecaps.pdf
Close