Label: SSD CREAM- silver sulfadiazine cream
- NDC Code(s): 43598-210-25, 43598-210-40, 43598-210-50, 43598-210-55, view more
- Packager: Dr Reddys Laboratories Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 24, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
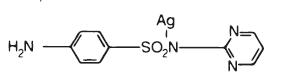
DESCRPITIONSSD™ (1% Silver Sulfadiazine Cream) and SSD AF™ (1% Silver Sulfadiazine Cream), 1% are topical antibacterial preparations which - have as their active ...
-
CLINICAL PHARMACOLOGYSilver sulfadiazine has broad antimicrobial activity. It is - bactericidal for many gram-negative and gram-positive bacteria as well as being - effective ...
-
INDICATIONS AND USAGESilver Sulfadiazine Cream is a topical antimicrobial drug indicated as - an adjunct for the prevention and treatment of wound sepsis in patients with - second ...
-
CONTRAINDICATIONSSilver Sulfadiazine Cream is contraindicated in patients who are - hypersensitive to silver sulfadiazine or any of the other ingredients in the ...
-
WARNINGThere is a potential cross-sensitivity between silver sulfadiazine and - other sulfonamides. If allergic reactions attributable to treatment with - silver ...
-
PRECAUTIONIf hepatic and renal functions become impaired and elimination of the - drug decreases accumulation may occur and discontinuation of Silver - Sulfadiazine ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term dermal toxicity studies of 24 months duration in rats and 18 - months in mice with concentrations of silver sulfadiazine three to ten times - the ...
-
ADVERSE REACTIONSSeveral cases of transient leucopenia have been reported in patients - receiving silver sulfadiazine therapy. Leucopenia associated with silver ...
-
DOSAGE AND ADMINISTRATION: FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE:Prompt institution of appropriate regimens for care of the burned - patient is of prime importance and includes the control of shock and pain. The - burn ...
-
HOW SUPPLIEDSSD™ (1% Silver Sulfadiazine) Cream: white to off-white cream. 50 gram jar NDC 43598-210-55 - 400 gram jar NDC 43598-210-40 - 25 gram tube ...
-
Container Label for 25 g TubeSSD™ NDC 43598-210-25 - Dr. Reddy’s - 25 Grams - 1% Silver Sulfadiazine Cream 1% FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE - Rx Only - Silver Sulfadiazine…10mg/g - in a hydrophilic base consisting ...
-
Container Label for 50 g TubeSSD™ NDC 43598-210-50 - Dr. Reddy’s - 50 Grams - 1% Silver Sulfadiazine Cream 1% FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE - Rx Only - Silver Sulfadiazine…10mg/g - in a hydrophilic base consisting ...
-
Container Label for 85 g TubeSSD™ NDC 43598-210-85 - Dr. Reddy’s - 85 Grams - 1% Silver Sulfadiazine Cream 1% FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE - Rx Only - Silver Sulfadiazine…10mg/g - in a hydrophilic base consisting ...
-
Container Label for 50 g JarSSD™ NDC 43598-210-55 - Dr. Reddy’s - 85 Grams - 1% Silver Sulfadiazine Cream 1% FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE - Rx Only - Silver Sulfadiazine…10mg/g - in a hydrophilic base consisting ...
-
Container Label for 400 g JarSSD™ NDC 43598-210-40 - Dr. Reddy’s - 85 Grams - 1% Silver Sulfadiazine Cream 1% FOR TOPICAL USE ONLY - NOT FOR OPHTHALMIC USE - Rx Only - Silver Sulfadiazine…10mg/g - in a hydrophilic base consisting ...
-
INGREDIENTS AND APPEARANCEProduct Information