Label: OLMESARTAN MEDOXOMIL tablet
- NDC Code(s): 43547-299-03, 43547-299-09, 43547-299-50, 43547-300-03, view more
- Packager: Solco Healthcare US, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OLMESARTAN MEDOXOMIL TABLETS safely and effectively. See full prescribing information for OLMESARTAN MEDOXOMIL TABLETS. Initial U.S ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE Olmesartan medoxomil is indicated for the treatment of hypertension in adults and children six years of age and older, to lower blood pressure. Lowering blood pressure reduces the risk of fatal ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Adult Hypertension Dosage must be individualized. The usual recommended starting dose of olmesartan medoxomil tablets is 20 mg once daily when used as monotherapy in patients who are not ...
-
3 DOSAGE FORMS AND STRENGTHS • 5 mg pink, round, film-coated, non-scored tablets debossed with S299 on one side and plain on the other side - • 20 mg white, round, film-coated, non-scored tablets debossed with S300 on one ...
-
4 CONTRAINDICATIONS Do not co-administer aliskiren with olmesartan medoxomil tablets in patients with diabetes [see Drug Interactions (7.3)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Fetal Toxicity Olmesartan medoxomil tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system (RAS) during the second and ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS 7.1 Agents Increasing Serum Potassium Concomitant use of olmesartan with other agents that block the renin-angiotensin system, potassium-sparing diuretics (e.g., spironolactone, triamterene ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk Summary - Olmesartan medoxomil tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second ...
-
10 OVERDOSAGE Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could be encountered if parasympathetic ...
-
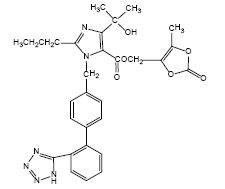
11 DESCRIPTION Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan is a selective AT1 subtype angiotensin II receptor antagonist ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Olmesartan medoxomil was not carcinogenic when administered by dietary administration to rats for up to 2 years. The highest dose ...
-
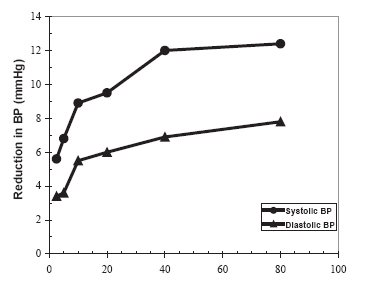
14 CLINICAL STUDIES 14.1 Adult Hypertension - The antihypertensive effects of olmesartan medoxomil tablets have been demonstrated in seven placebo-controlled studies at doses ranging from 2.5 mg to 80 mg for 6 to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Olmesartan medoxomil tablets, USP, are supplied as pink, round, film-coated, non-scored tablets containing 5 mg of olmesartan medoxomil, as white, round, film-coated, non-scored tablets containing ...
-
17 PATIENT COUNSELING INFORMATION Pregnancy: Advise female patients of childbearing age about the consequences of exposure to olmesartan medoxomil tablets during pregnancy. Discuss treatment options with women planning to become ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label-5 mg-30 tablets - Rx only - NDC 43547-299-03 - Olmesartan Medoxomil Tablets, USP - Each tablet contains: Active ingredient: olmesartan medoxomil USP, 5 mg. Store at 20 - 25°C (68 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label- 20 mg-30 tablets - Rx only - NDC 43547-300-03 - Olmesartan Medoxomil Tablets, USP - Each tablet contains: Active ingredient: olmesartan medoxomil USP, 20 mg. Store at 20 - 25°C (68 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label- 40 mg-30 tablets - Rx only - NDC 43547-301-03 - Olmesartan Medoxomil Tablets, USP - Each tablet contains: Active ingredient: olmesartan medoxomil USP, 40 mg. Store at 20 - 25°C (68 ...
-
INGREDIENTS AND APPEARANCEProduct Information