Label: DONEPEZIL HYDROCHLORIDE tablet, film coated
- NDC Code(s): 43547-275-03, 43547-275-09, 43547-275-11, 43547-276-03, view more
- Packager: Solco Healthcare US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DONEPEZIL HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for DONEPEZIL HYDROCHLORIDE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDonepezil hydrochloride is indicated for the treatment of dementia of the Alzheimer's type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer's disease.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing in Mild to Moderate Alzheimer's Disease - The recommended starting dosage of donepezil hydrochloride is 5 mg administered once per day in the evening, just prior to retiring. The ...
-
3 DOSAGE FORMS AND STRENGTHSDonepezil hydrochloride is supplied as film-coated, round tablets containing 5 mg, 10 mg of donepezil hydrochloride, USP. The 5 mg tablets are blue, round, film-coated tablets, debossed with ...
-
4 CONTRAINDICATIONSDonepezil hydrochloride is contraindicated in patients with known hypersensitivity to donepezil hydrochloride or to piperidine derivatives.
-
5 WARNINGS AND PRECAUTIONS5.1 Anesthesia - Donepezil hydrochloride, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia. 5.2 Cardiovascular ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: • Cardiovascular Conditions [see Warnings and Precautions (5.2)] • Nausea and Vomiting [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Use with Anticholinergics - Because of their mechanism of action, cholinesterase inhibitors have the potential to interfere with the activity of anticholinergic medications. 7.2 Use ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risks associated with the use of donepezil hydrochloride in pregnant women. In animal studies, developmental ...
-
10 OVERDOSAGEBecause strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an ...
-
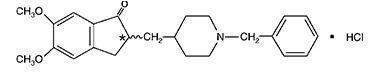
11 DESCRIPTIONDonepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Current theories on the pathogenesis of the cognitive signs and symptoms of Alzheimer's disease attribute some of them to a deficiency of cholinergic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of carcinogenic potential was obtained in an 88-week carcinogenicity study of donepezil conducted in mice at oral doses up ...
-
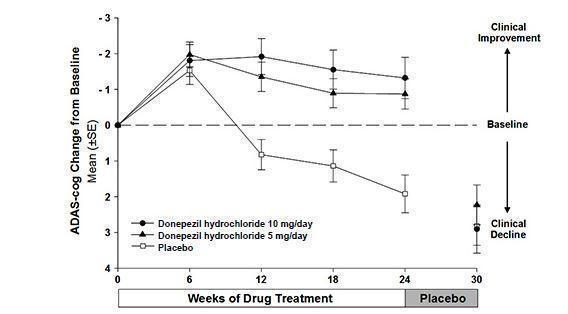
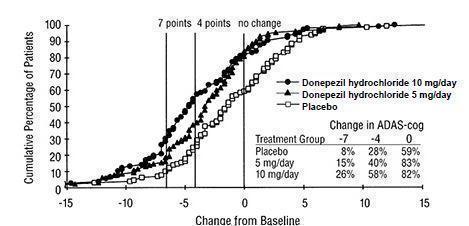
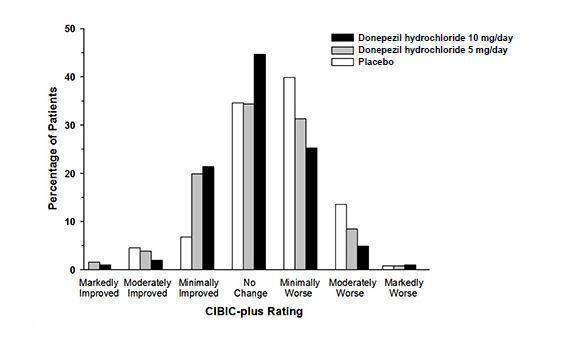
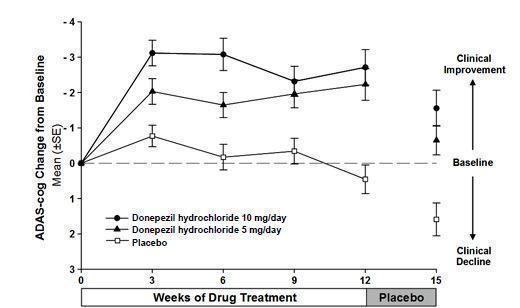
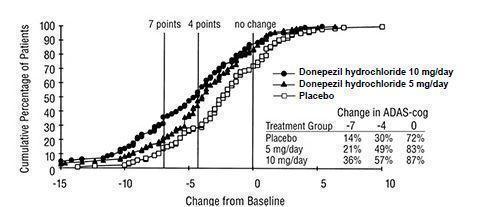
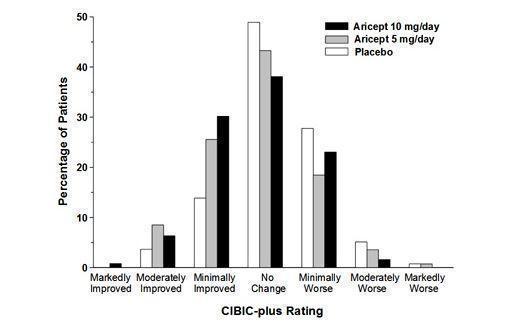
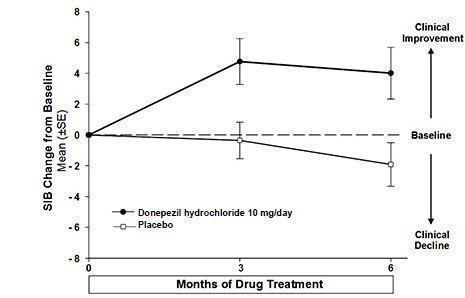
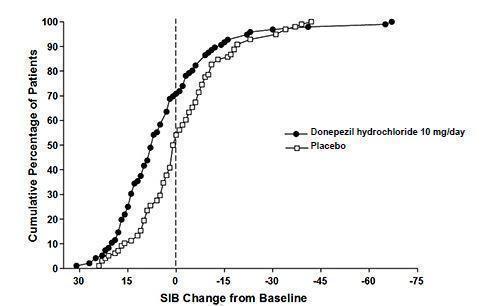
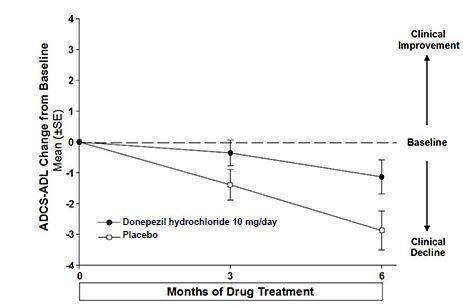
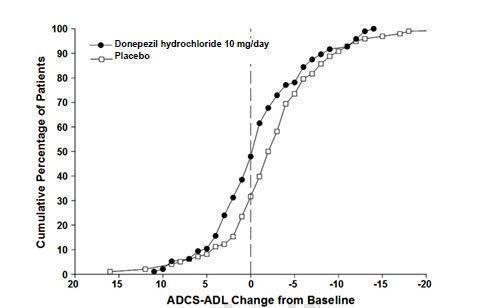
14 CLINICAL STUDIES14.1 Mild to Moderate Alzheimer's Disease - The effectiveness of donepezil hydrochloride as a treatment for mild to moderate Alzheimer's disease is demonstrated by the results of two randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 Donepezil Hydrochloride Tablets - Supplied as film-coated, round tablets containing 5 mg, or 10 mg of donepezil hydrochloride, USP. The 5 mg tablets are blue, round, film-coated tablets ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Instruct patients and caregivers to take donepezil hydrochloride only once per day, as prescribed. Instruct ...
-
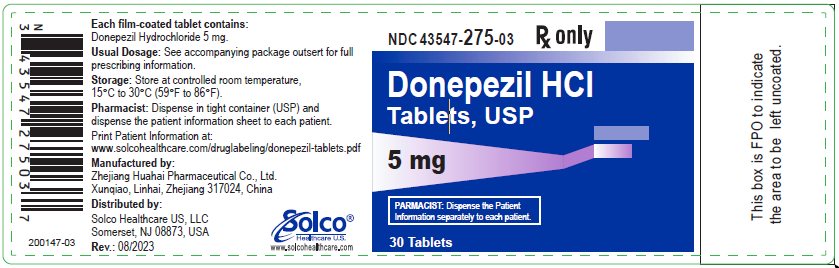
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 5 mg Bottle Label-Existing Manufacturing Site ZhejiangHuahai Xunqiao - NDC 43547-275-03 Rx only - Donepezil HCI - Tablets, USP - 5 mg - 30 ...
-
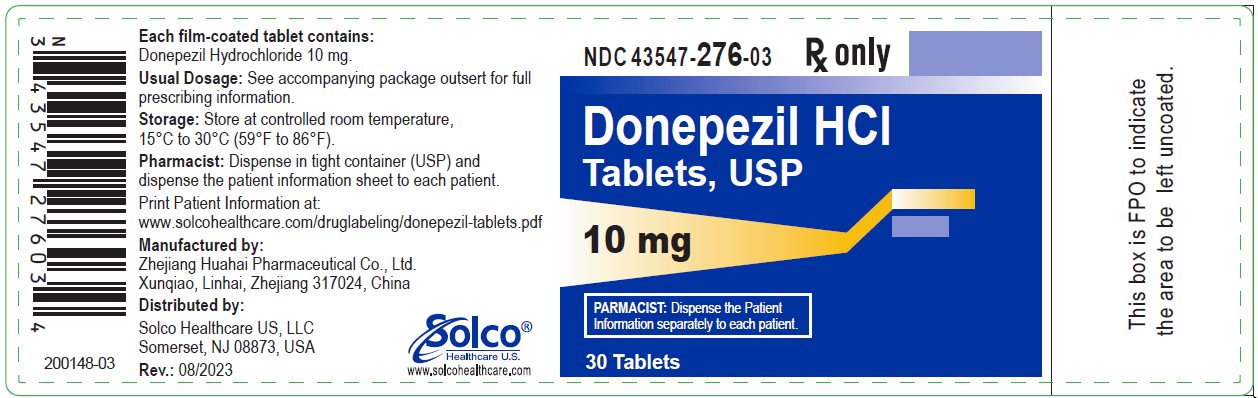
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 10 mg Bottle Label-Existing Manufacturing Site Zhejiang Huahai Xunqiao - NDC 43547-276-03 Rx only - Donepezil HCI - Tablets, USP - 10 mg - 30 ...
-
Principal Display Panel – 5 mg Bottle Label- Alternate Manufacturing Site Zhejiang Huahai Technology Jiangnan NDC 43547-275-03 Rx only - Donepezil HCI - Tablets, USP - 5 mg - 30 Tablets - Solco - Healthcare U.S.
-
Principal Display Panel – 10 mg Bottle Label- Alternate Manufacturing Site Zhejiang Huahai Technology Jiangnan NDC 43547-276-03 Rx only - Donepezil HCI - Tablets, USP - 10 mg - 30 Tablets - Solco - Healthcare U.S.
-
INGREDIENTS AND APPEARANCEProduct Information