Label: GAVILAX- polyethylene glycol 3350 powder, for solution
- NDC Code(s): 43386-312-07, 43386-312-08, 43386-312-14, 43386-312-85
- Packager: Lupin Pharmaceuticals,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientPolyethylene Glycol 3350, 17 g (cap filled to line)……………………Osmotic Laxative
-
PurposeUse - relieves occasional constipation (irregularity) generally produces a bowel movement in 1 to 3 days
-
WARNINGSAllergy alert: Do not use if you are allergic to polyethylene glycol - Do not use if you have kidney disease, except under the advice and supervision of a doctor
-
Ask a doctor before use if you havenausea, vomiting or abdominal pain - a sudden change in bowel habits that lasts over 2 weeks - irritable bowel syndrome - Ask a doctor or pharmacist before use if you are taking a prescription drug ...
-
Stop use and ask a doctor ifyou have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition. you get diarrhea - you need to use a laxative for longer than 1 ...
-
Keep out of the reach of childrenIn case of overdose, get medical help or contact a POISON CONTROL CENTER right away. (1-800-222-1222)
-
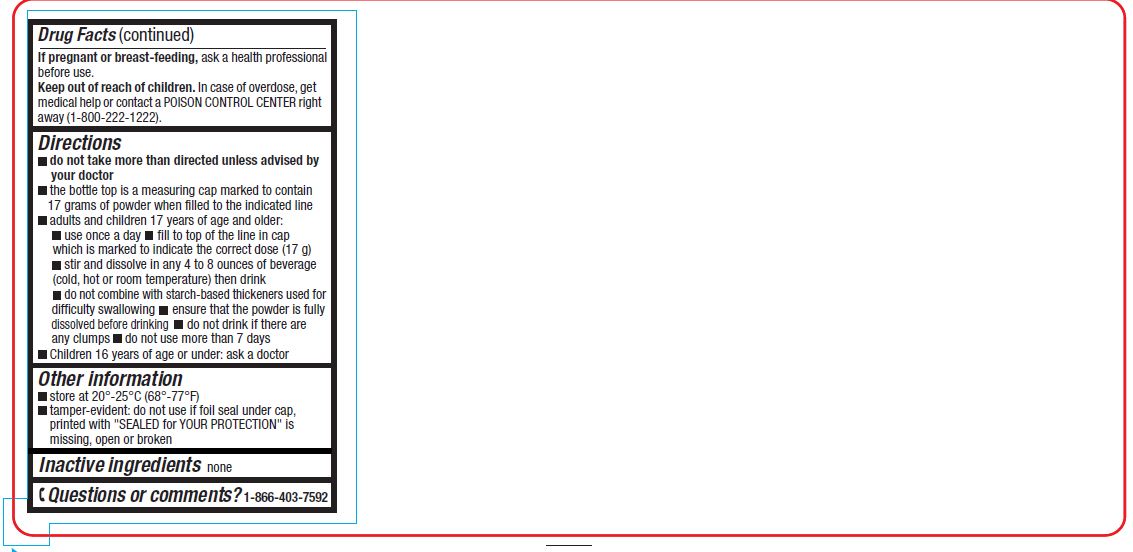
Directionsdo not take more than directed unless advised by your doctor - the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line. adults and children 17 ...
-
Inactive Ingredientnone
-
Questions or comments?1-866-403-7592 - Dissolves in any beverages - Sugar Free
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 43386-312-08 - Original Prescription Strength - GaviLax - Polyethylene Glycol 3350 - Powder for Oral Solution, Osmotic Laxative - 14 ONCE-DAILY DOSES - NET WT 8.3 OZ (238 g) NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information