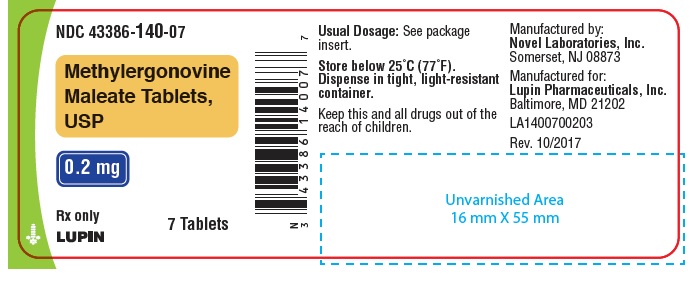
Label: METHYLERGONOVINE MALEATE tablet
-
Contains inactivated NDC Code(s)
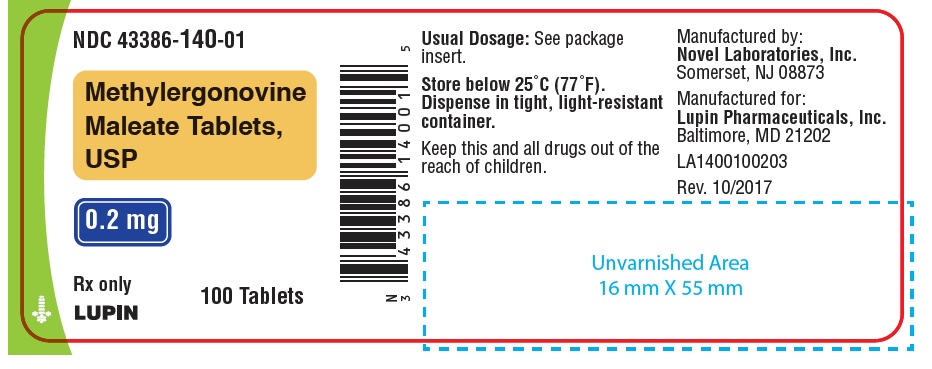
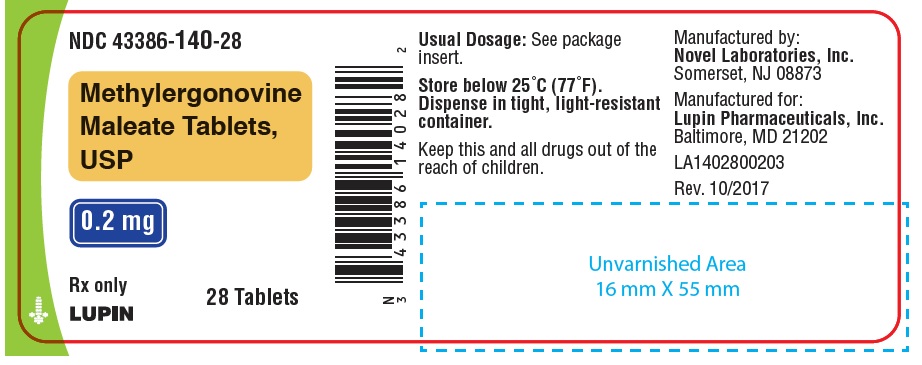
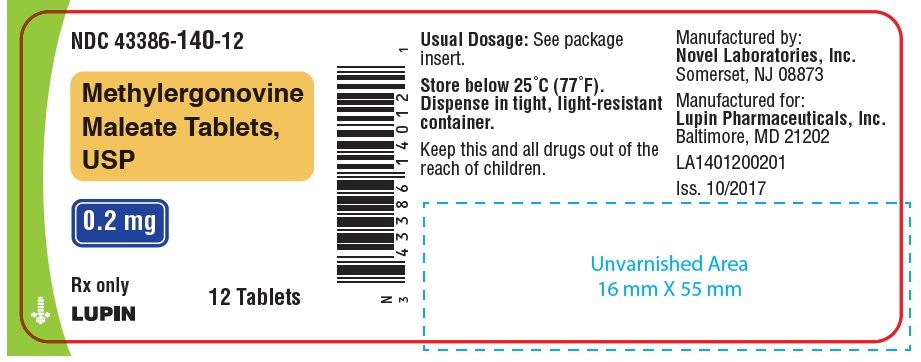
NDC Code(s): 43386-140-01, 43386-140-07, 43386-140-12, 43386-140-28 - Packager: Lupin Pharmaceuticals,Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 13, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
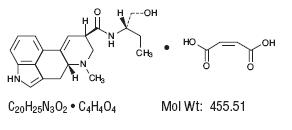
DESCRIPTIONMethylergonovine Maleate is a semi-synthetic ergot alkaloid used for the prevention and control of postpartum hemorrhage. Methylergonovine Maleate Tablets, USP is available in tablets for oral ...
-
CLINICAL PHARMACOLOGYMethylergonovine maleate acts directly on the smooth muscle of the uterus and increases the tone, rate, and amplitude of rhythmic contractions. Thus, it induces a rapid and sustained tetanic ...
-
INDICATIONS AND USAGEFollowing delivery of placenta, for routine management of uterine atony, hemorrhage and subinvolution of the uterus. For control of uterine hemorrhage in the second stage of labor following ...
-
CONTRAINDICATIONSHypertension; toxemia; pregnancy; and hypersensitivity.
-
WARNINGSGeneral - This drug should not be administered I.V. routinely because of the possibility of inducing sudden hypertensive and cerebrovascular accidents. If I.V administration is considered ...
-
PRECAUTIONSGeneral - Caution should be exercised in the presence of sepsis, obliterative vascular disease. Also use with caution during the second stage of labor. The necessity for manual removal of a ...
-
ADVERSE REACTIONSThe most common adverse reaction is hypertension associated in several cases with seizure and/or headache. Hypotension has also been reported. Abdominal pain (caused by uterine contractions) ...
-
DRUG ABUSE AND DEPENDENCEMethylergonovine maleate has not been associated with drug abuse or dependence of either a physical or psychological nature.
-
OVERDOSAGESymptoms of acute overdose may include: nausea, vomiting, oliquria, abdominal pain, numbness, tingling of the extremities, rise in blood pressure, in severe cases followed by hypotension ...
-
DOSAGE AND ADMINISTRATIONParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Intramuscularly - 1 mL, 0.2 mg, after delivery of the anterior shoulder ...
-
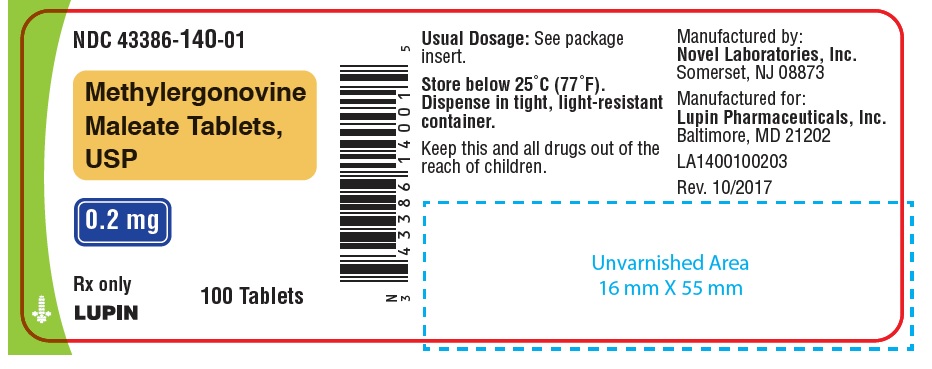
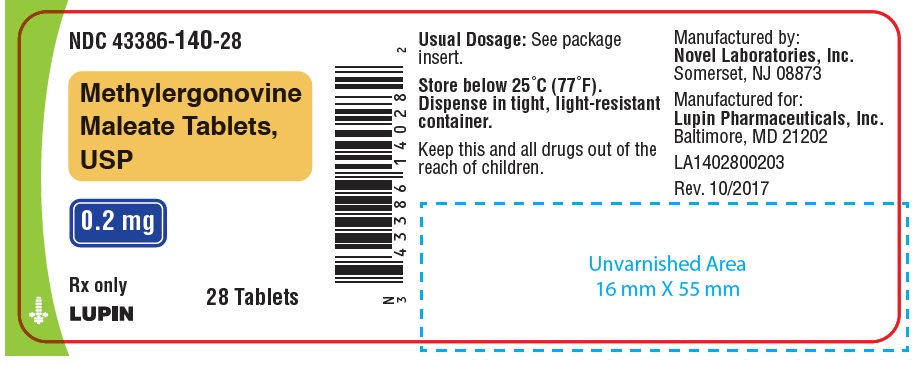
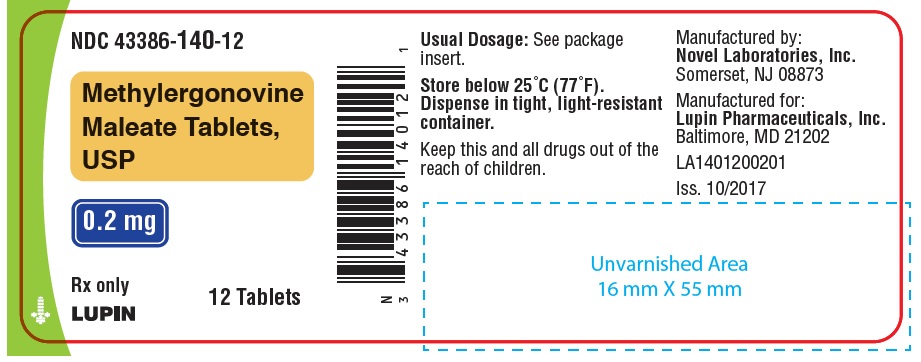
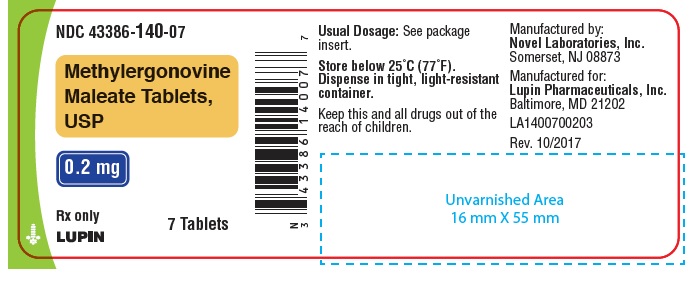
HOW SUPPLIEDWhite, round, biconvex compressed tablets debossed with "n" on one side and "01" on the other side. Available in bottles of 7, 12, 28 and 100 tablets. Bottles of 7 ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 43386-140-01 - Rx Only - Methylergonovine Maleate Tablets, USP - 0.2 mg - 100 Tablets - NDC 43386-140-28 - Rx Only - Methylergonovine Maleate Tablets, USP - 0.2 mg - 28 Tablets - NDC 43386-140-12 - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information