Label: FLECAINIDE ACETATE tablet
- NDC Code(s): 42806-817-01, 42806-818-01, 42806-819-01
- Packager: Epic Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
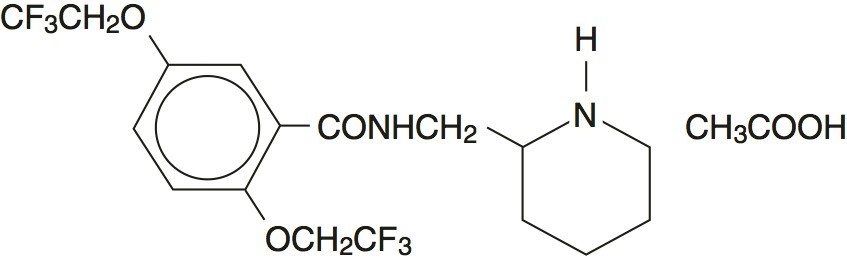
DESCRIPTIONFlecainide Acetate Tablets, USP are an antiarrhythmic drug containing 50 mg, 100 mg or 150 mg of flecainide acetate USP for oral administration. Flecainide acetate USP is benzamide ...
-
CLINICAL PHARMACOLOGYFlecainide acetate has local anesthetic activity and belongs to the membrane stabilizing (Class 1) group of antiarrhythmic agents; it has electrophysiologic effects characteristic of the IC class ...
-
INDICATIONS AND USAGEIn patients without structural heart disease, Flecainide Acetate Tablets, USP are indicated for the prevention of: paroxysmal supraventricular tachycardias (PSVT), including atrioventricular ...
-
CONTRAINDICATIONSFlecainide Acetate Tablets are contraindicated in patients with pre-existing second- or third-degree AV block, or with right bundle branch block when associated with a left hemiblock (bifascicular ...
-
WARNINGSMortality - Flecainide acetate was included in the National Heart Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicenter, randomized, double-blind ...
-
PROARRHYTHMIC EFFECTSFlecainide acetate, like other antiarrhythmic agents, can cause new or worsened supraventricular or ventricular arrhythmias. Ventricular proarrhythmic effects range from an increase in frequency ...
-
HEART FAILUREFlecainide acetate has a negative inotropic effect and may cause or worsen CHF, particularly in patients with cardiomyopathy, preexisting severe heart failure (NYHA functional class III or IV) or ...
-
PRECAUTIONSDrug Interactions - Flecainide acetate has been administered to patients receiving - digitalispreparations or - beta-adrenergic blocking agentswithout adverse effects. During administration ...
-
ADVERSE REACTIONSIn post-myocardial infarction patients with asymptomatic PVCs and non-sustained ventricular tachycardia, flecainide acetate therapy was found to be associated with a 5.1% rate of death and ...
-
OVERDOSAGENo specific antidote has been identified for the treatment of flecainide acetate overdosage. Overdoses ranging up to 8,000 mg have been survived, with peak plasma flecainide concentrations as high ...
-
DOSAGE AND ADMINISTRATIONFor patients with - sustainedVT, no matter what their cardiac status, Flecainide Acetate Tablets, USP like other antiarrhythmics, should be initiated in-hospital with rhythm monitoring ...
-
HOW SUPPLIEDFlecainide Acetate Tablets, USP - 50 mg tablet is supplied as a white biconvex round tablet with “YH 777” debossed on one side and plain on the other side. Bottles of 100 tablets with ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELFlecainide Acetate Tablets, USP - 50 mg, 100 Tablets - NDC: 42806-817-01
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELFlecainide Acetate Tablets, USP - 100 mg, 100 Tablets - NDC: 42806-818-01
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELFlecainide Acetate Tablets, USP - 150 mg, 100 Tablets - NDC: 42806-819-01
-
INGREDIENTS AND APPEARANCEProduct Information