Label: BENZONATATE capsule
- NDC Code(s): 42806-714-01, 42806-714-05, 42806-715-01, 42806-715-05
- Packager: Epic Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
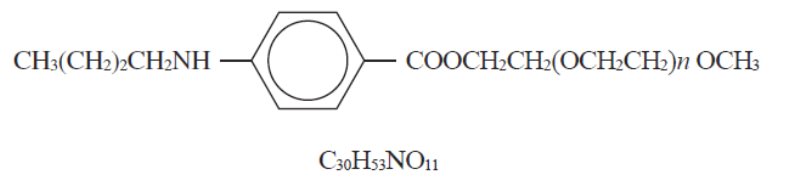
Benzonatate capsules, USP, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26- onaoxaoctacosan-28-ylp-(butylamino) benzoate; with a molecular weight of 603.7. Each ...
-
CLINICAL PHARMACOLOGYBenzonatate capsules act peripherally by anesthetizing the stretch receptors located in the respiratory passages, lungs, and pleura by dampening their activity and thereby reducing the cough ...
-
INDICATIONS AND USAGEBenzonatate capsules, USP are indicated for the symptomatic relief of cough.
-
CONTRAINDICATIONSHypersensitivity to benzonatate or related compounds.
-
WARNINGSHypersensitivity - Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from ...
-
PRECAUTIONSBenzonatate is chemically related to anesthetic agents of the para-amino-benzoic acid class (e.g. procaine; tetracaine) and has been associated with adverse CNS effects possibly related to a prior ...
-
ADVERSE REACTIONSPotential Adverse Reactions to benzonatate capsules may include: Hypersensitivity reactionsincluding bronchospasm, laryngospasm, cardiovascular collapse possibly related to local anesthesia from ...
-
OVERDOSAGEIntentional and unintentional overdose may result in death, particularly in children. The drug is chemically related to tetracaine and other topical anesthetics and shares various aspects of their ...
-
DOSAGE AND ADMINISTRATIONAdults and Children over 10 years of age: Usual dose is one 100 mg or 200 mg capsule three times a day as needed for cough. If necessary to control cough, up to 600 mg daily in three divided doses ...
-
HOW SUPPLIEDBenzonatate Capsules USP, 100 mg are clear yellow, oval-shaped softgel capsules imprinted with “PC14” and are supplied as follows: NDC 42806-714-01 in bottle of 100 capsules - NDC 42806-714-05 in ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 100 mg 500ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 200 mg 500ct

-
INGREDIENTS AND APPEARANCEProduct Information



 Each benzonatate capsules, USP for oral administration contains 100 mg or 200 mg benzonatate, USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow #10, gelatin, glycerin, lecithin, light mineral oil, propylene glycol, purified water, shellac glaze, titanium dioxide, and white edible ink.
Each benzonatate capsules, USP for oral administration contains 100 mg or 200 mg benzonatate, USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow #10, gelatin, glycerin, lecithin, light mineral oil, propylene glycol, purified water, shellac glaze, titanium dioxide, and white edible ink.