Mechanism of Action
- Nicardipine hydrochloride capsules are a calcium entry blocker (slow channel blocker or calcium ion antagonist) that inhibits the transmembrane influx of calcium ions into ...
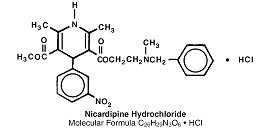
Mechanism of Action
Nicardipine hydrochloride capsules are a calcium entry blocker (slow channel blocker or calcium ion antagonist) that inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle without changing serum calcium concentrations. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. The effects of nicardipine hydrochloride capsules are more selective to vascular smooth muscle than cardiac muscle. In animal models, Nicardipine hydrochloride capsules produce relaxation of coronary vascular smooth muscle at drug levels that cause little or no negative inotropic effect.
Pharmacokinetics and Metabolism
Nicardipine hydrochloride capsules are completely absorbed following oral doses administered as capsules. Plasma levels are detectable as early as 20 minutes following an oral dose and maximal plasma levels are observed within 30 minutes to 2 hours (mean T max = 1 hour). While nicardipine hydrochloride capsules are completely absorbed, it is subject to saturable first pass metabolism and the systemic bioavailability is about 35% following a 30-mg oral dose at steady-state.
When nicardipine hydrochloride capsules were administered 1 or 3 hours after a high-fat meal, the mean C max and mean AUC were lower (20% to 30%) than when nicardipine hydrochloride capsules were given to fasting subjects. These decreases in plasma levels observed following a meal may be significant, but the clinical trials establishing the efficacy and safety of nicardipine hydrochloride capsules were done in patients without regard to the timing of meals. Thus, the results of these trials reflect the effects of meal-induced variability.
The pharmacokinetics of nicardipine hydrochloride capsules are nonlinear due to saturable hepatic first pass metabolism. Following oral administration, increasing doses result in a disproportionate increase in plasma levels. Steady-state C max values following 20-, 30-, and 40-mg doses every 8 hours averaged 36, 88, and 133 ng/mL, respectively. Hence, increasing the dose from 20 to 30 mg every 8 hours more than doubled C max and increasing the dose from 20 to 40 mg every 8 hours increased C max more than threefold. A similar disproportionate increase in AUC with dose was observed. Considerable inter-subject variability in plasma levels was also observed.
Post-absorption kinetics of nicardipine hydrochloride capsules are also non-linear, although there is a reproducible terminal plasma half-life that averaged 8.6 hours following 30- and 40-mg doses at steady-state (tid). The terminal half-life represents the elimination of less than 5% of the absorbed drug (measured by plasma concentrations). Elimination over the first 8 hours after dosing is much faster with a half-life of 2 to 4 hours. Steady-state plasma levels are achieved after 2 to 3 days of tid dosing (every 8 hours) and are twofold higher than after a single dose.
Nicardipine hydrochloride capsules are highly protein bound (>95%) in human plasma over a wide concentration range.
Nicardipine hydrochloride capsules are metabolized extensively by the hepatic cytochrome P450 enzymes, CYP2C8, 2D6, and 3A4; less than 1% of intact drug is detected in the urine. Following a radioactive oral dose in solution, 60% of the radioactivity was recovered in the urine and 35% in feces. Most of the dose (over 90%) was recovered within 48 hours of dosing. Nicardipine hydrochloride capsules do not induce its own metabolism, however, nicardipine causes inhibition of certain cytochrome P450 enzymes (including CYP3A4, CYP2D6, CYP2C8, and CYP2C19). Inhibition of these enzymes may result in increased plasma levels of certain drugs, including cyclosporine and tacrolimus ( see Drug Interactions). The altered pharmacokinetics may necessitate dosage adjustment of the affected drug or discontinuation of treatment.
Nicardipine hydrochloride plasma levels were higher in patients with mild renal impairment (baseline serum creatinine concentration ranged from 1.2 to 5.5 mg/dL) than in normal subjects. After 30-mg nicardipine hydrochloride tid at steady-state, C max and AUC were approximately twofold higher in these patients.
Because nicardipine hydrochloride capsules are extensively metabolized by the liver, the plasma levels of the drug are influenced by changes in hepatic function. Nicardipine hydrochloride plasma levels were higher in patients with severe liver disease (hepatic cirrhosis confirmed by liver biopsy or presence of endoscopically-confirmed esophageal varices) than in normal subjects. After 20-mg nicardipine hydrochloride capsules bid at steady-state, C max and AUC were 1.8 and fourfold higher, and the terminal half-life was prolonged to 19 hours in these patients.
Geriatric Pharmacokinetics
The steady-state pharmacokinetics of nicardipine hydrochloride capsules in elderly hypertensive patients (≥65 years) are similar to those obtained in young normal adults. After 1 week of nicardipine hydrochloride capsules dosing at 20 mg three times a day, the C max, T max, AUC, terminal plasma half-life and the extent of protein binding of nicardipine hydrochloride capsules observed in healthy elderly hypertensive patients did not differ significantly from those observed in young normal volunteers
Hemodynamics
In man, nicardipine hydrochloride capsules produce a significant decrease in systemic vascular resistance. The degree of vasodilation and the resultant hypotensive effects are more prominent in hypertensive patients. In hypertensive patients, nicardipine reduces the blood pressure at rest and during isometric and dynamic exercise. In normotensive patients, a small decrease of about 9 mm Hg in systolic and 7 mm Hg in diastolic blood pressure may accompany this fall in peripheral resistance. An increase in heart rate may occur in response to the vasodilation and decrease in blood pressure, and in a few patients this heart rate increase may be pronounced. In clinical studies mean heart rate at time of peak plasma levels was usually increased by 5 to 10 beats per minute compared to placebo, with the greater increases at higher doses, while there was no difference from placebo at the end of the dosing interval. Hemodynamic studies following intravenous dosing in patients with coronary artery disease and normal or moderately abnormal left ventricular function have shown significant increases in ejection fraction and cardiac output with no significant change, or a small decrease, in left ventricular end-diastolic pressure (LVEDP). Although there is evidence that nicardipine hydrochloride capsules increase coronary blood flow, there is no evidence that this property plays any role in its effectiveness in stable angina. In patients with coronary artery disease, intracoronary administration of nicardipine caused no direct myocardial depression. Nicardipine hydrochloride capsules do, however, have a negative inotropic effect in some patients with severe left ventricular dysfunction and could, in patients with very impaired function, lead to worsened failure.
“Coronary Steal”, the detrimental redistribution of coronary blood flow in patients with coronary artery disease (diversion of blood from underperfused areas toward better perfused areas), has not been observed during nicardipine treatment. On the contrary, nicardipine has been shown to improve systolic shortening in normal and hypokinetic segments of myocardial muscle, and radio-nuclide angiography has confirmed that wall motion remained improved during an increase in oxygen demand. Nonetheless, occasional patients have developed increased angina upon receiving nicardipine. Whether this represents steal in those patients, or is the result of increased heart rate and decreased diastolic pressure, is not clear.
In patients with coronary artery disease nicardipine improves L.V. diastolic distensibility during the early filling phase, probably due to a faster rate of myocardial relaxation in previously underperfused areas. There is little or no effect on normal myocardium, suggesting the improvement is mainly by indirect mechanisms such as afterload reduction, and reduced ischemia. Nicardipine has no negative effect on myocardial relaxation at therapeutic doses. The clinical consequences of these properties are as yet undemonstrated.
Electrophysiologic Effects
In general, no detrimental effects on the cardiac conduction system were seen with the use of nicardipine hydrochloride capsules.
Nicardipine hydrochloride increased the heart rate when given intravenously during acute electrophysiologic studies and prolonged the corrected QT interval to a minor degree. The sinus node recovery times and SA conduction times were not affected by the drug. The PA, AH, and HV intervals 1 and the functional and effective refractory periods of the atrium were not prolonged by nicardipine hydrochloride capsules and the relative and effective refractory periods of the His-Purkinje system were slightly shortened after intravenous nicardipine hydrochloride.
1PA = conduction time from high to low right atrium, AH = conduction time from low right atrium to His bundle deflection or AV nodal conduction time, HV = conduction time through the His bundle and the bundle branch-Purkinje system.
Renal Function
There is a transient increase in electrolyte excretion, including sodium. Nicardipine hydrochloride capsules do not cause generalized fluid retention, as measured by weight changes, although 7% to 8% of the patients experience pedal edema.
Effects in Angina Pectoris
In controlled clinical trials of up to 12 weeks’ duration in patients with chronic stable angina, nicardipine hydrochloride capsules increased exercise tolerance and reduced nitroglycerin consumption and the frequency of anginal attacks. The antianginal efficacy of nicardipine hydrochloride capsules (20 to 40 mg) have been demonstrated in four placebo-controlled studies involving 258 patients with chronic stable angina. In exercise tolerance testing, nicardipine hydrochloride capsules significantly increased time to angina, total exercise duration and time to 1 mm ST segment depression. Included among these four studies was a dose-definition study in which dose-related improvements in exercise tolerance at 1 and 4 hours postdosing and reduced frequency of anginal attacks were seen at doses of 10, 20 and 30 mg tid. Effectiveness at 10 mg tid was, however, marginal. In a fifth placebo-controlled study, the antianginal efficacy of nicardipine hydrochloride capsules were demonstrated at 8 hours postdose (trough). The sustained efficacy of nicardipine hydrochloride capsules have been demonstrated over long-term dosing. Blood pressure fell in patients with angina by about 10/8 mm Hg at peak blood levels and was little different from placebo at trough blood levels.
Effects in Hypertension
Nicardipine hydrochloride capsules produced dose-related decreases in both systolic and diastolic blood pressure in clinical trials. The antihypertensive efficacy of nicardipine hydrochloride capsules administered three times daily has been demonstrated in three placebo-controlled studies involving 517 patients with mild to moderate hypertension. The blood pressure responses in the three studies were statistically significant from placebo at peak (1 hour postdosing) and trough (8 hours postdosing) although it is apparent that well over half of the antihypertensive effect is lost by the end of the dosing interval. The results from placebo controlled studies of nicardipine hydrochloride capsules given three times daily are shown in the following table:
The responses are shown as differences from the concurrent placebo control group. The large changes between peak and trough effects were not accompanied by observed side effects at peak response times. In a study using 24-hour intra-arterial blood pressure monitoring, the circadian variation in blood pressure remained unaltered, but the systolic and diastolic blood pressures were reduced throughout the whole 24 hours.
When added to beta-blocker therapy, nicardipine hydrochloride capsules further lower both systolic and diastolic blood pressure.
Close