Label: LEUCOVORIN CALCIUM tablet
- NDC Code(s): 42806-133-21, 42806-133-24, 42806-134-24, 42806-358-01, view more
- Packager: Epic Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
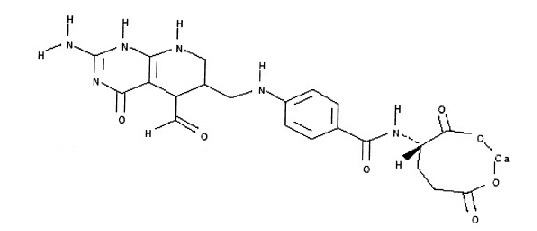
Leucovorin calcium tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of ...
-
CLINICAL PHARMACOLOGY Leucovorin is a racemic mixture of the diastereoisomers of the 5-formyl derivative of tetrahydrofolic acid. The biologically active compound of the mixture is the (-)-L-isomer, known as Citrovorum ...
-
INDICATIONS AND USAGE Leucovorin calcium tablets are indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosages of folic acid antagonists.
-
CONTRAINDICATIONS Leucovorin is improper therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of Vitamin B12. A hematologic remission may occur while neurologic manifestations ...
-
WARNINGS In the treatment of accidental overdosage of folic acid antagonists, leucovorin should be administered as promptly as possible. As the time interval between antifolate administration (e.g. ...
-
PRECAUTIONS General - Parenteral administration is preferable to oral dosing if there is a possibility that the patient may vomit or not absorb the leucovorin. Leucovorin has no effect on other established ...
-
ADVERSE REACTIONS Allergic sensitization, including anaphylactoid reactions and urticaria, has been reported following the administration of both oral and parenteral leucovorin.
-
OVERDOSAGE Excessive amounts of leucovorin may nullify the chemotherapeutic effect of folic acid antagonists.
-
DOSAGE AND ADMINISTRATION Leucovorin calcium tablets are intended for oral administration. Because absorption is saturable, oral administration of doses greater than 25 mg is not recommended. Impaired Methotrexate ...
-
HOW SUPPLIED Leucovorin Calcium Tablets USP, 5 mg are off-white, round biconvex tablets, debossed “Є” above “358” on one side and bisected on the other side and are supplied as follows. NDC ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Leucovorin Calcium Tablets USP, 5 mg 100ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Leucovorin Calcium Tablets USP, 10 mg 12ct
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Leucovorin Calcium Tablets USP, 15 mg 24ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Leucovorin Calcium Tablets USP, 25 mg 25ct

-
INGREDIENTS AND APPEARANCEProduct Information