Label: BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE tablet, film coated
-
NDC Code(s):
42799-920-01,
42799-920-02,
42799-920-30,
42799-921-01, view more42799-921-02, 42799-921-30, 42799-922-01, 42799-922-02, 42799-922-30
- Packager: Edenbridge Pharmaceuticals LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONBisoprolol Fumarate and Hydrochlorothiazide Tablets, USP - Rx only - Revised January 2024 - DESCRIPTION - Bisoprolol fumarate and hydrochlorothiazide tablets, USP, are indicated for the ...
Bisoprolol Fumarate and Hydrochlorothiazide Tablets, USP
Rx only
Revised January 2024
DESCRIPTIONBisoprolol fumarate and hydrochlorothiazide tablets, USP, are indicated for the treatment of hypertension. It combines two antihypertensive agents in a once-daily dosage: a synthetic beta1-selective (cardioselective) adrenoceptor blocking agent (bisoprolol fumarate) and a benzothiadiazine diuretic (hydrochlorothiazide).
Bisoprolol fumarate is chemically described as (±)-1-[4-[[2-(1 methylethoxy)ethoxy]methyl] phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-butenedioate (2:1) (salt). It possesses an asymmetric carbon atom in its structure and is provided as a racemic mixture. The S(-) enantiomer is responsible for most of the beta-blocking activity. Its empirical formula is (C18H31NO4)2•C4H4O4 and it has a molecular weight of 766.97. Its structural formula is:
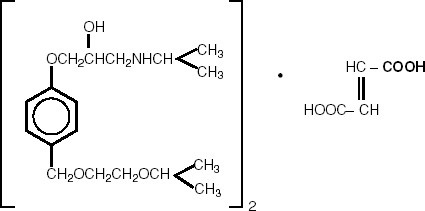
Bisoprolol fumarate is a white crystalline powder, approximately equally hydrophilic and lipophilic, and readily soluble in water, methanol, ethanol, and chloroform.
Hydrochlorothiazide (HCTZ) is 6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. It is a white, or practically white, practically odorless crystalline powder. It is slightly soluble in water, sparingly soluble in dilute sodium hydroxide solution, freely soluble in n-butylamine and dimethylformamide, sparingly soluble in methanol, and insoluble in ether, chloroform, and dilute mineral acids. Its empirical formula is C7H8ClN3O4S2 and it has a molecular weight of 297.73. Its structural formula is:
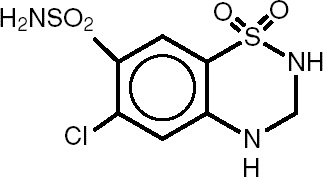
Each bisoprolol fumarate and hydrochlorothiazide tablet 2.5 mg/6.25 mg for oral administration contains:
Bisoprolol fumarate....................................................... 2.5 mg
Hydrochlorothiazide….................................................... 6.25 mg
Each bisoprolol fumarate and hydrochlorothiazide tablet 5 mg/6.25 mg for oral administration contains:
Bisoprolol fumarate...................................................…. 5 mg
Hydrochlorothiazide….................................................... 6.25 mg
Each bisoprolol fumarate and hydrochlorothiazide tablet 10 mg/6.25 mg for oral administration contains:
Bisoprolol fumarate...................................................... 10 mg
Hydrochlorothiazide….................................................... 6.25 mg
Inactive ingredients include Anhydrous Lactose, Crospovidone, Hypromellose, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 80, Pregelatinized Starch, Stearic Acid, and Titanium Dioxide. The 5 mg/6.25 mg tablet also contains D&C Red #30. The 2.5 mg/6.25 mg tablet also contains D&C Yellow #10 Aluminum Lake and FD&C Yellow #6.
Close -
CLINICAL PHARMACOLOGYBisoprolol fumarate and HCTZ have been used individually and in combination for the treatment of hypertension. The antihypertensive effects of these agents are additive; HCTZ 6.25 mg significantly ...
Bisoprolol fumarate and HCTZ have been used individually and in combination for the treatment of hypertension. The antihypertensive effects of these agents are additive; HCTZ 6.25 mg significantly increases the antihypertensive effect of bisoprolol fumarate. The incidence of hypokalemia with the bisoprolol fumarate and HCTZ 6.25 mg combination (B/H) is significantly lower than with HCTZ 25 mg. In clinical trials of bisoprolol fumarate and hydrochlorothiazide tablets, mean changes in serum potassium for patients treated with bisoprolol fumarate and hydrochlorothiazide tablets 2.5/6.25 mg, 5/6.25 mg or 10/6.25 mg or placebo were less than ± 0.1 mEq/L. Mean changes in serum potassium for patients treated with any dose of bisoprolol in combination with HCTZ 25 mg ranged from -0.1 to -0.3 mEq/L.
Bisoprolol fumarate is a beta1-selective (cardioselective) adrenoceptor blocking agent without significant membrane stabilizing or intrinsic sympathomimetic activities in its therapeutic dose range. At higher doses (≥ 20 mg) bisoprolol fumarate also inhibits beta2-adrenoreceptors located in bronchial and vascular musculature. To retain relative selectivity, it is important to use the lowest effective dose.
Hydrochlorothiazide is a benzothiadiazine diuretic. Thiazides affect renal tubular mechanisms of electrolyte reabsorption and increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium.
Pharmacokinetics and Metabolism
Bisoprolol fumarate and hydrochlorothiazide tablets
In healthy volunteers, both bisoprolol fumarate and hydrochlorothiazide are well absorbed following oral administration of bisoprolol fumarate and hydrochlorothiazide tablets. No change is observed in the bioavailability of either agent when given together in a single tablet. Absorption is not affected whether bisoprolol fumarate and hydrochlorothiazide tablets is taken with or without food. Mean peak bisoprolol fumarate plasma concentrations of about 9.0 ng/mL, 19 ng/mL and 36 ng/mL occur approximately 3 hours after the administration of the 2.5 mg/6.25 mg, 5 mg/6.25 mg and 10 mg/6.25 mg combination tablets, respectively. Mean peak plasma hydrochlorothiazide concentrations of 30 ng/mL occur approximately 2.5 hours following the administration of the combination. Dose proportional increases in plasma bisoprolol concentrations are observed between the 2.5 and 5, as well as between the 5 and 10 mg doses.
The elimination T1/2 of bisoprolol ranges from 7 to 15 hours, and that of hydrochlorothiazide ranges from 4 to 10 hours. The percent of dose excreted unchanged in urine is about 55% for bisoprolol and about 60% for hydrochlorothiazide.
Bisoprolol Fumarate
The absolute bioavailability after a 10 mg oral dose of bisoprolol fumarate is about 80%. The first pass metabolism of bisoprolol fumarate is about 20%.The pharmacokinetic profile of bisoprolol fumarate has been examined following single doses and at steady state. Binding to serum proteins is approximately 30%. Peak plasma concentrations occur within 2-4 hours of dosing with 2.5 to 20 mg, and mean peak values range from 9.0 ng/mL at 2.5 mg to 70 ng/mL at 20 mg. Once-daily dosing with bisoprolol fumarate results in less than twofold intersubject variation in peak plasma concentrations. Plasma concentrations are proportional to the administered dose in the range of 2.5 to 20 mg. The plasma elimination half- life is 9-12 hours and is slightly longer in elderly patients, in part because of decreased renal function. Steady state is attained within 5 days with once-daily dosing. In both young and elderly populations, plasma accumulation is low; the accumulation factor ranges from 1.1 to 1.3, and is what would be expected from the half-life and once-daily dosing. Bisoprolol is eliminated equally by renal and nonrenal pathways with about 50% of the dose appearing unchanged in the urine and the remainder in the form of inactive metabolites. In humans, the known metabolites are labile or have no known pharmacologic activity. Less than 2% of the dose is excreted in the feces. The pharmacokinetic characteristics of the two enantiomers are similar. Bisoprolol is not metabolized by cytochrome P450 II D6 (debrisoquin hydroxylase).
In subjects with creatinine clearance less than 40 mL/min, the plasma half-life is increased approximately threefold compared to healthy subjects.
In patients with liver cirrhosis, the rate of elimination of bisoprolol is more variable and significantly slower than that in healthy subjects, with a plasma half-life ranging from 8 to 22 hours.
In elderly subjects, mean plasma concentrations at steady state are increased, in part attributed to lower creatinine clearance. However, no significant differences in the degree of bisoprolol accumulation is found between young and elderly populations.
Hydrochlorothiazide
Hydrochlorothiazide is well absorbed (65%-75%) following oral administration. Absorption of hydrochlorothiazide is reduced in patients with congestive heart failure.
Peak plasma concentrations are observed within 1-5 hours of dosing, and range from 70-490 ng/mL following oral doses of 12.5-100 mg. Plasma concentrations are linearly related to the administered dose. Concentrations of hydrochlorothiazide are 1.6-1.8 times higher in whole blood than in plasma. Binding to serum proteins has been reported to be approximately 40% to 68%. The plasma elimination half-life has been reported to be 6-15 hours. Hydrochlorothiazide is eliminated primarily by renal pathways. Following oral doses of 12.5-100 mg, 55%-77% of the administered dose appears in urine and greater than 95% of the absorbed dose is excreted in urine as unchanged drug. Plasma concentrations of hydrochlorothiazide are increased, and the elimination half-life is prolonged in patients with renal disease.
ClosePharmacodynamics
Bisoprolol Fumarate
Findings in clinical hemodynamics studies with bisoprolol fumarate are similar to those observed with other beta-blockers. The most prominent effect is the negative chronotropic effect, giving a reduction in resting and exercise heart rate. There is a fall in resting and exercise cardiac output with little observed change in stroke volume, and only a small increase in right atrial pressure, or pulmonary capillary wedge pressure at rest or during exercise.
In normal volunteers, bisoprolol fumarate therapy resulted in a reduction of exercise- and isoproterenol-induced tachycardia. The maximal effect occurred within 1-4 hours post-dosing. Effects generally persisted for 24 hours at doses of 5 mg or greater.
In controlled clinical trials, bisoprolol fumarate given as a single daily dose has been shown to be an effective antihypertensive agent when used alone or concomitantly with thiazide diuretics (see CLINICAL STUDIES). The mechanism of bisoprolol fumarate’s antihypertensive effect has not been completely established. Factors that may be involved include:
1) Decreased cardiac output,
2) Inhibition of renin release by the kidneys,
3) Diminution of tonic sympathetic outflow from vasomotor centers in the brain.
Beta1-selectivity of bisoprolol fumarate has been demonstrated in both animal and human studies. No effects at therapeutic doses on beta2-adrenoreceptor density have been observed. Pulmonary function studies have been conducted in healthy volunteers, asthmatics, and patients with chronic obstructive pulmonary disease (COPD). Doses of bisoprolol fumarate ranged from 5 to 60 mg, atenolol from 50 to 200 mg, metoprolol from 100 to 200 mg, and propranolol from 40 to 80 mg. In some studies, slight, asymptomatic increases in airway resistance (AWR) and decreases in forced expiratory volume (FEV1) were observed with doses of bisoprolol fumarate 20 mg and higher, similar to the small increases in AWR noted with other cardioselective beta-blocking agents. The changes induced by beta-blockade with all agents were reversed by bronchodilator therapy.
Electrophysiology studies in man have demonstrated that bisoprolol fumarate significantly decreases heart rate, increases sinus node recovery time, prolongs AV node refractory periods, and, with rapid atrial stimulation, prolongs AV nodal conduction.
Hydrochlorothiazide
Acute effects of thiazides are thought to result from a reduction in blood volume and cardiac output, secondary to a natriuretic effect, although a direct vasodilatory mechanism has also been proposed. With chronic administration, plasma volume returns toward normal, but peripheral vascular resistance is decreased.
Thiazides do not affect normal blood pressure. Onset of action occurs within 2 hours of dosing, peak effect is observed at about 4 hours, and activity persists for up to 24 hours.
-
CLINICAL STUDIESIn controlled clinical trials, bisoprolol fumarate/hydrochlorothiazide 6.25 mg has been shown to reduce systolic and diastolic blood pressure throughout a 24-hour period when administered once ...
In controlled clinical trials, bisoprolol fumarate/hydrochlorothiazide 6.25 mg has been shown to reduce systolic and diastolic blood pressure throughout a 24-hour period when administered once daily. The effects on systolic and diastolic
blood pressure reduction of the combination of bisoprolol fumarate and hydrochlorothiazide were additive. Further, treatment effects were consistent across age groups (<60, ≥ 60 years), racial groups (black, nonblack), and gender (male,
female).
In two randomized, double-blind, placebo-controlled trials conducted in the U.S., reductions in systolic and diastolic blood pressure and heart rate 24 hours after dosing in patients with mild-to moderate hypertension are shown below. In both studies mean systolic/diastolic blood pressure and heart rate at baseline were approximately 151/101 mm Hg and 77 bpm.
Close
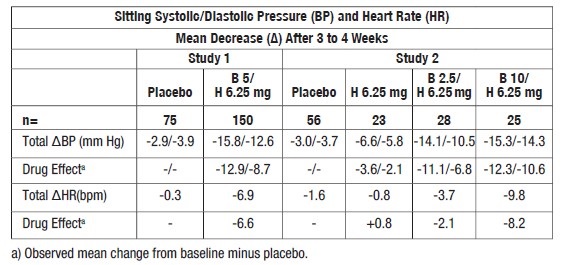
Blood pressure responses were seen within 1 week of treatment but the maximum effect was apparent after 2 to 3 weeks of treatment. Overall, significantly greater blood pressure reductions were observed on bisoprolol fumarate and hydrochlorothiazide tablets than on placebo. Further, blood pressure reductions were significantly greater for each of the bisoprolol fumarate plus hydrochlorothiazide combinations than for either of the components used alone regardless of race, age, or gender. There were no significant differences in response between black and nonblack patients. -
INDICATIONS & USAGEBisoprolol fumarate and hydrochlorothiazide tablets are indicated in the management of hypertension.
Bisoprolol fumarate and hydrochlorothiazide tablets are indicated in the management of hypertension.
Close -
CONTRAINDICATIONSBisoprolol fumarate and hydrochlorothiazide tablets are contraindicated in patients in cardiogenic shock, overt cardiac failure (see WARNINGS), second or third degree AV block, marked sinus ...
Bisoprolol fumarate and hydrochlorothiazide tablets are contraindicated in patients in cardiogenic shock, overt cardiac failure (see WARNINGS), second or third degree AV block, marked sinus bradycardia, anuria, and hypersensitivity to either component of this product or to other sulfonamide-derived drugs.
Close -
WARNINGSCardiac Failure - In general, beta-blocking agents should be avoided in patients with overt congestive failure. However, in some patients with compensated cardiac failure, it may be necessary to ...
Cardiac Failure
In general, beta-blocking agents should be avoided in patients with overt congestive failure. However, in some patients with compensated cardiac failure, it may be necessary to utilize these agents. In such situations, they must be used cautiously.
Patients Without a History of Cardiac Failure
Continued depression of the myocardium with beta-blockers can, in some patients, precipitate cardiac failure. At the first signs or symptoms of heart failure, discontinuation of bisoprolol fumarate and hydrochlorothiazide tablets should be considered. In some cases bisoprolol fumarate and hydrochlorothiazide tablet therapy can be continued while heart failure is treated with other drugs.
Abrupt Cessation of Therapy
Exacerbations of angina pectoris and, in some instances, myocardial infarction or ventricular arrhythmia, have been observed in patients with coronary artery disease following abrupt cessation of therapy with beta-blockers. Such patients should, therefore, be cautioned against interruption or discontinuation of therapy without the physician’s advice. Even in patients without overt coronary artery disease, it may be advisable to taper therapy with bisoprolol fumarate and hydrochlorothiazide tablets over approximately 1 week with the patient under careful observation. If withdrawal symptoms occur, beta-blocking agent therapy should be reinstituted, at least temporarily.
Peripheral Vascular Disease
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
Bronchospastic Disease
PATIENTS WITH BRONCHOSPASTIC PULMONARY DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of the relative beta1-selectivity of bisoprolol fumarate, bisoprolol fumarate and hydrochlorothiazide tablets may be used with caution in patients with bronchospastic disease who do not respond to, or who cannot tolerate other antihypertensive treatment. Since beta1-selectivity is not absolute, the lowest possible dose of bisoprolol fumarate and hydrochlorothiazide tablets should be used. A beta2 agonist (bronchodilator) should be made available.
Major SurgeryChronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Hypoglycemia
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment. Also, latent diabetes mellitus may become manifest and diabetic patients given thiazides may require adjustment of their insulin dose.
Thyrotoxicosis
Beta-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate thyroid storm.
Renal Disease
Cumulative effects of the thiazides may develop in patients with impaired renal function. In such patients, thiazides may precipitate azotemia. In subjects with creatinine clearance less than 40 mL/min, the plasma half-life of bisoprolol fumarate is increased up to threefold, as compared to healthy subjects. If progressive renal impairment becomes apparent, bisoprolol fumarate and hydrochlorothiazide tablets should be discontinued (See Pharmacokinetics and Metabolism).
Hepatic Disease
Bisoprolol fumarate and hydrochlorothiazide tablets should be used with caution in patients with impaired hepatic function or progressive liver disease. Thiazides may alter fluid and electrolyte balance, which may precipitate hepatic coma. Also, elimination of bisoprolol fumarate is significantly slower in patients with cirrhosis than in healthy subjects (See Pharmacokinetics and Metabolism).
Acute Angle-Closure Glaucoma with or without Acute Myopia and Choroidal Effusions
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute angle-closure glaucoma and elevated intraocular pressure with or without a noticeable acute myopic shift and/or choroidal effusions. Symptoms may include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated, the acute angle-closure glaucoma may result in permanent visual field loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Close -
PRECAUTIONSGENERAL PRECAUTIONS - Electrolyte and Fluid Balance Status - Although the probability of developing hypokalemia is reduced with bisoprolol fumarate and hydrochlorothiazide tablets because of ...
GENERAL PRECAUTIONS
Electrolyte and Fluid Balance Status
Although the probability of developing hypokalemia is reduced with bisoprolol fumarate and hydrochlorothiazide tablets because of the very low dose of HCTZ employed, periodic determination of serum electrolytes should be performed, and patients should be observed for signs of fluid or electrolyte disturbances, i.e., hyponatremia, hypochloremic alkalosis, hypokalemia, and hypomagnesemia. Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Warning signs or symptoms of fluid and electrolyte imbalance include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis when severe cirrhosis is present, during concomitant use of corticosteroids or adrenocorticotropic hormone (ACTH) or after prolonged therapy. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia and hypomagnesemia can provoke ventricular arrhythmias or sensitize or exaggerate the response of the heart to the toxic effects of digitalis. Hypokalemia may be avoided or treated by potassium supplementation or increased intake of potassium-rich foods.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than salt administration, except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Parathyroid Disease
Calcium excretion is decreased by thiazides, and pathologic changes in the parathyroid glands, with hypercalcemia and hypophosphatemia, have been observed in a few patients on prolonged thiazide therapy.
Hyperuricemia
Hyperuricemia or acute gout may be precipitated in certain patients receiving thiazide diuretics. Bisoprolol fumarate, alone or in combination with HCTZ, has been associated with increases in uric acid. However, in U.S. clinical trials, the incidence of treatment-related increases in uric acid was higher during therapy with HCTZ 25 mg (25%) than with B/H 6.25 mg (10%). Because of the very low dose of HCTZ employed, hyperuricemia may be less likely with bisoprolol fumarate and hydrochlorothiazide tablets.
Drug Interactions
Bisoprolol fumarate and hydrochlorothiazide tablets may potentiate the action of other antihypertensive agents used concomitantly. Bisoprolol fumarate and hydrochlorothiazide tablets should not be combined with other beta-blocking agents. Patients receiving catecholamine- depleting drugs, such as reserpine or guanethidine, should be closely monitored because the added beta-adrenergic blocking action of bisoprolol fumarate may produce excessive reduction of sympathetic activity. In patients receiving concurrent therapy with clonidine, if therapy is to be discontinued, it is suggested that bisoprolol fumarate and hydrochlorothiazide tablets be discontinued for several days before the withdrawal of clonidine.
Bisoprolol fumarate and hydrochlorothiazide tablets should be used with caution when myocardial depressants or inhibitors of AV conduction, such as certain calcium antagonists (particularly of the phenylalkylamine [verapamil] and benzothiazepine [diltiazem] classes), or antiarrhythmic agents, such as disopyramide, are used concurrently.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Bisoprolol Fumarate
Concurrent use of rifampin increases the metabolic clearance of bisoprolol fumarate, shortening its elimination half-life. However, initial dose modification is generally not necessary.
Pharmacokinetic studies document no clinically relevant interactions with other agents given concomitantly, including thiazide diuretics and cimetidine. There was no effect of bisoprolol fumarate on prothrombin times in patients on stable doses of warfarin.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
Hydrochlorothiazide
When given concurrently the following drugs may interact with thiazide diuretics.
Alcohol, barbiturates, or narcotics - potentiation of orthostatic hypotension may occur.
Antidiabetic drugs (oral agents and insulin) - dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs - additive effect or potentiation.
Cholestyramine and colestipol resins - Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of cholestyramine and colestipol resins bind the hydrochlorothiazide and reduce its absorption in the gastrointestinal tract by up to 85 percent and 43 percent, respectively.
Corticosteroids, ACTH - Intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine) - possible decreased response to pressor amines but not sufficient to preclude their use.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) - possible increased responsiveness to the muscle relaxant.
Lithium - generally should not be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with bisoprolol fumarate and hydrochlorothiazide tablets.
Nonsteroidal anti-inflammatory drugs - In some patients, the administration of a nonsteroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium sparing, and thiazide diuretics. Therefore, when bisoprolol fumarate and hydrochlorothiazide tablets and nonsteroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
In patients receiving thiazides, sensitivity reactions may occur with or without a history of allergy or bronchial asthma. Photosensitivity reactions and possible exacerbation or activation of systemic lupus erythematosus have been reported in patients receiving thiazides. The antihypertensive effects of thiazides may be enhanced in the post-sympathectomy patient.
Laboratory Test Interactions
Based on reports involving thiazides, bisoprolol fumarate and hydrochlorothiazide tablets may decrease serum levels of protein-bound iodine without signs of thyroid disturbance.
Because it includes a thiazide, bisoprolol fumarate and hydrochlorothiazide tablets should be discontinued before carrying out tests for parathyroid function (see PRECAUTIONS- Parathyroid Disease).
INFORMATION FOR PATIENTS
Warn patients, especially those with coronary artery disease, against discontinuing use of bisoprolol fumarate and hydrochlorothiazide tablets without a physician’s supervision. Patients should also be advised to consult a physician if any difficulty in breathing occurs, or if they develop other signs or symptoms of congestive heart failure or excessive bradycardia.
Inform patients or caregivers that there is a risk of hypoglycemia when bisoprolol fumarate and hydrochlorothiazide tablets is given to patients who are fasting or who are vomiting. Monitor for symptoms of hypoglycemia.
Patients should know how they react to this medicine before they operate automobiles and machinery or engage in other tasks requiring alertness.
Advise patients that photosensitivity reactions have been reported with thiazides.Non-melanoma Skin Cancer
Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Acute Angle-Closure Glaucoma
Instruct patients taking hydrochlorothiazide to immediately consult their healthcare provider if visual field defects, decreased visual acuity, or ocular pain occur.CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis
Bisoprolol fumarate and hydrochlorothiazide tablets
Long-term studies have not been conducted with the bisoprolol fumarate/hydrochlorothiazide combination.
Bisoprolol Fumarate
Long-term studies were conducted with oral bisoprolol fumarate administered in the feed of mice (20 and 24 months) and rats (26 months). No evidence of carcinogenic potential was seen in mice dosed up to 250 mg/kg/day or rats dosed up to 125 mg/kg/day. On a body weight basis, these doses are 625 and 312 times, respectively, the maximum recommended human dose (MRHD) of 20 mg, or 0.4 mg/kg/day, based on 50 kg individuals; on a body surface area basis, these doses are 59 times (mice) and 64 times (rats) the MRHD.
Hydrochlorothiazide
Two-year feeding studies in mice and rats, conducted under the auspices of the National Toxicology Program (NTP), treated mice and rats with doses of hydrochlorothiazide up to 600 and 100 mg/kg/day, respectively. On a body weight basis, these doses are 2400 times (in mice) and 400 times (in rats) the MRHD of hydrochlorothiazide (12.5 mg/day) in bisoprolol fumarate and hydrochlorothiazide tablets. On a body surface area basis, these doses are 226 times (in mice) and 82 times (in rats) the MRHD. These studies uncovered no evidence of carcinogenic potential of hydrochlorothiazide in rats or female mice, but there was equivocal evidence of hepatocarcinogenicity in male mice.
Mutagenesis
Bisoprolol fumarate and hydrochlorothiazide tablets
The mutagenic potential of the bisoprolol fumarate/hydrochlorothiazide combination was evaluated in the microbial mutagenicity (Ames) test, the point mutation and chromosomal aberration assays in Chinese hamster V79 cells, and the micronucleus test in mice. There was no evidence of mutagenic potential in these in vitro and in vivo assays.
Bisoprolol Fumarate
The mutagenic potential of bisoprolol fumarate was evaluated in the microbial mutagenicity (Ames) test, the point mutation and chromosome aberration assays in Chinese hamster V79 cells, the unscheduled DNA synthesis test, the micronucleus test in mice, and the cytogenetics assay in rats. There was no evidence of mutagenic potential in these in vitro and in vivo assays.
Hydrochlorothiazide
Hydrochlorothiazide was not genotoxic in in vitro assays using strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538 of Salmonella typhimurium (the Ames test); in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations; or in in vivo assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained in the in vitro CHO Sister Chromatid Exchange (clastogenicity) test and in the mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide of 43-1300 µg/mL. Positive test results were also obtained in the Aspergillus nidulans non-disjunction assay, using an unspecified concentration of hydrochlorothiazide.
Impairment of Fertility
Bisoprolol fumarate and hydrochlorothiazide tablets
Reproduction studies in rats did not show any impairment of fertility with the bisoprolol fumarate/hydrochlorothiazide combination doses containing up to 30 mg/kg/day of bisoprolol fumarate in combination with 75 mg/kg/day of hydrochlorothiazide. On a body weight basis, these doses are 75 and 300 times, respectively, the MRHD of bisoprolol fumarate and hydrochlorothiazide. On a body surface area basis, these study doses are 15 and 62 times, respectively, MRHD.
Bisoprolol Fumarate
Reproduction studies in rats did not show any impairment of fertility at doses up to 150 mg/kg/day of bisoprolol fumarate, or 375 and 77 times the MRHD on the basis of body weight and body surface area, respectively.
Hydrochlorothiazide
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg/day, respectively, prior to mating and throughout gestation. Corresponding multiples of maximum recommended human doses are 400 (mice) and 16 (rats) on the basis of body weight and 38 (mice) and 3.3 (rats) on the basis of body surface area.
PREGNANCY
Teratogenic Effects
Bisoprolol fumarate and hydrochlorothiazide tablets
In rats, the bisoprolol fumarate/hydrochlorothiazide (B/H) combination was not teratogenic at doses up to 51.4 mg/kg/day of bisoprolol fumarate in combination with 128.6 mg/kg/day of hydrochlorothiazide. Bisoprolol fumarate and hydrochlorothiazide doses used in the rat study are, as multiples of the MRHD in the combination, 129 and 514 times greater, respectively, on a body weight basis, and 26 and 106 times greater, respectively, on the basis of body surface area. The drug combination was maternotoxic (decreased body weight and food consumption) at B5.7/H14.3 (mg/kg/day) and higher, and fetotoxic (increased late resorptions) at B17.1/H42.9 (mg/kg/day) and higher. Maternotoxicity was present at 14/57 times the MRHD of B/H, respectively, on a body weight basis, and 3/12 times the MRHD of B/H doses, respectively, on the basis of body surface area. Fetotoxicity was present at 43/172 times the MRHD of B/H, respectively, on a body weight basis, and 9/35 times the MRHD of B/H doses, respectively, on the basis of body surface area. In rabbits, the B/H combination was not teratogenic at doses of B10/H25 (mg/kg/day). Bisoprolol fumarate and hydrochlorothiazide used in the rabbit study were not teratogenic at 25/100 times the B/H MRHD, respectively, on a body weight basis, and 10/40 times the B/H MRHD, respectively, on the basis of body surface area. The drug combination was maternotoxic (decreased body weight) at B1/H2.5 (mg/kg/day) and higher, and fetotoxic (increased resorptions) at B10/H25 (mg/kg/day). The multiples of the MRHD for the B/H combination that were maternotoxic are, respectively, 2.5/10 (on the basis of body weight) and 1/4 (on the basis of body surface area), and for fetotoxicity were, respectively 25/100 (on the basis of body weight) and 10/40 (on the basis of body surface area).
There are no adequate and well-controlled studies with bisoprolol fumarate and hydrochlorothiazide tablets in pregnant women. Bisoprolol fumarate and hydrochlorothiazide tablets should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Bisoprolol Fumarate
In rats, bisoprolol fumarate was not teratogenic at doses up to 150 mg/kg/day, which were 375 and 77 times the MRHD on the basis of body weight and body surface area, respectively. Bisoprolol fumarate was fetotoxic (increased late resorptions) at 50 mg/kg/day and maternotoxic (decreased food intake and body weight gain) at 150 mg/kg/day. The fetotoxicity in rats occurred at 125 times the MRHD on a body weight basis and 26 times the MRHD on the basis of body surface area. The maternotoxicity occurred at 375 times the MRHD on a body weight basis and 77 times the MRHD on the basis of body surface area. In rabbits, bisoprolol fumarate was not teratogenic at doses up to 12.5 mg/kg/day, which is 31 and 12 times the MRHD based on body weight and body surface area, respectively, but was embryolethal (increased early resorptions) at 12.5 mg/kg/day.
Hydrochlorothiazide
Hydrochlorothiazide was orally administered to pregnant mice and rats during respective periods of major organogenesis at doses up to 3000 and 1000 mg/kg/day, respectively. At these doses, which are multiples of the MRHD equal to 12,000 for mice and 4000 for rats, based on body weight, and equal to 1129 for mice and 824 for rats, based on body surface area, there was no evidence of harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.NONTERATOGENIC EFFECTS
Thiazides cross the placental barrier and appear in the cord blood. The use of thiazides in pregnant women requires that the anticipated benefit be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, pancreatitis, thrombocytopenia, and possibly other adverse reactions that have occurred in the adult.
NURSING MOTHERS
Bisoprolol fumarate alone or in combination with HCTZ has not been studied in nursing mothers. Thiazides are excreted in human breast milk. Small amounts of bisoprolol fumarate (<2% of the dose) have been detected in the milk of lactating rats. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
PEDIATRIC USE
Safety and effectiveness of bisoprolol fumarate and hydrochlorothiazide tablets in pediatric patients have not been established.
CloseGERIATRIC USE
In clinical trials, at least 270 patients treated with bisoprolol fumarate plus HCTZ were 60 years of age or older. HCTZ added significantly to the antihypertensive effect of bisoprolol in elderly hypertensive patients. No overall differences in effectiveness or safety were observed between these patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONSBisoprolol fumarate and hydrochlorothiazide tablets - Bisoprolol fumarate/HCTZ 6.25 mg is well tolerated in most patients. Most adverse effects (AEs) have been mild and transient. In more than ...
Bisoprolol fumarate and hydrochlorothiazide tablets
Bisoprolol fumarate/HCTZ 6.25 mg is well tolerated in most patients. Most adverse effects (AEs) have been mild and transient. In more than 65,000 patients treated worldwide with bisoprolol fumarate, occurrences of bronchospasm have been rare. Discontinuation rates for AEs were similar for bisoprolol fumarate/HCTZ 6.25 mg and placebo-treated patients.
In the United States, 252 patients received bisoprolol fumarate (2.5, 5, 10, or 40 mg)/HCTZ 6.25 mg and 144 patients received placebo in two controlled trials. In Study 1, bisoprolol fumarate 5/HCTZ 6.25 mg was administered for 4 weeks. In Study 2, bisoprolol fumarate 2.5, 10, or 40/HCTZ 6.25 mg was administered for 12 weeks. All adverse experiences, whether drug related or not, and drug related adverse experiences in patients treated with bisoprolol fumarate 2.5 10/HCTZ 6.25 mg, reported during comparable, 4 week treatment periods by at least 2% of bisoprolol fumarate/HCTZ 6.25 mg-treated patients (plus additional selected adverse experiences) are presented in the following table:
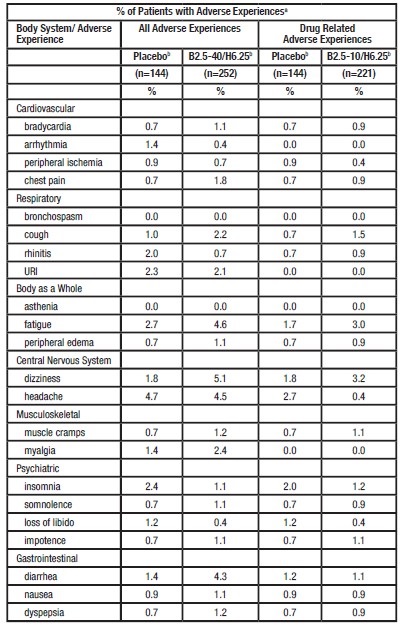
a) Averages adjusted to combine across studies.
b) Combined across studies.
Other adverse experiences that have been reported with the individual components are listed below.
Bisoprolol Fumarate
In clinical trials worldwide, or in postmarketing experience, a variety of other AEs, in addition to those listed above, have been reported. While in many cases it is not known whether a causal relationship exists between bisoprolol and these AEs, they are listed to alert the physician to a possible relationship.
Central Nervous System
Unsteadiness, dizziness, vertigo, headache, syncope, paresthesia, hypoesthesia, hyperesthesia, sleep disturbance/vivid dreams, insomnia, somnolence, depression, anxiety/restlessness, decreased concentration/memory.
Cardiovascular
Bradycardia, palpitations and other rhythm disturbances, cold extremities, claudication, hypotension, orthostatic hypotension, chest pain, congestive heart failure, dyspnea on exertion.
Gastrointestinal
Gastric/epigastric/abdominal pain, peptic ulcer, gastritis, dyspepsia, nausea, vomiting, diarrhea, constipation, dry mouth.
Musculoskeletal
Arthralgia, muscle/joint pain, back/neck pain, muscle cramps, twitching/tremor.
Skin
Rash, acne, eczema, psoriasis, skin irritation, pruritus, purpura, flushing, sweating, alopecia, dermatitis, exfoliative dermatitis (very rarely), cutaneous vasculitis.
Special Senses
Visual disturbances, ocular pain/pressure, abnormal lacrimation, tinnitus, decreased hearing, earache, taste abnormalities.
Metabolic
Gout.
Respiratory
Asthma, bronchospasm, bronchitis, dyspnea, pharyngitis, rhinitis, sinusitis, URI (upper respiratory infection).
Genitourinary
Decreased libido/impotence, Peyronie’s disease (very rarely), cystitis, renal colic, polyuria.
GeneralFatigue, asthenia, chest pain, malaise, edema, weight gain, angioedema.
In addition, a variety of adverse effects have been reported with other beta-adrenergic blocking agents and should be considered potential adverse effects:
Central Nervous System
Reversible mental depression progressing to catatonia, hallucinations, an acute reversible syndrome characterized by disorientation to time and place, emotional lability, slightly clouded sensorium.
Allergic
Fever, combined with aching and sore throat, laryngospasm, and respiratory distress.
Hematologic
Agranulocytosis, thrombocytopenia.
Gastrointestinal
Mesenteric arterial thrombosis and ischemic colitis.
Miscellaneous
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with bisoprolol fumarate during investigational use or extensive foreign marketing experience.
Hydrochlorothiazide
The following adverse experiences, in addition to those listed in the above table, have been reported with hydrochlorothiazide (generally with doses of 25 mg or greater).
General
Weakness.
Central Nervous SystemVertigo, paresthesia, restlessness.
Cardiovascular
Orthostatic hypotension (may be potentiated by alcohol, barbiturates, or narcotics).
GastrointestinalAnorexia, gastric irritation, cramping, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis, cholecystitis, sialadenitis, dry mouth.
Musculoskeletal
Muscle spasm.
Hypersensitive Reactions
Purpura, photosensitivity, rash, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions.
Special Senses
Transient blurred vision, choroidal effusion, xanthopsia.
MetabolicGout.
Genitourinary
Sexual dysfunction, renal failure, renal dysfunction, interstitial nephritis.
Skin
Erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis.
Postmarketing Experience
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.Laboratory Abnormalities
Bisoprolol fumarate and hydrochlorothiazide tablets
Because of the low dose of hydrochlorothiazide in bisoprolol fumarate and hydrochlorothiazide tablets, adverse metabolic effects with bisoprolol fumarate/HCTZ 6.25 mg are less frequent and of smaller magnitude than with HCTZ 25 mg. Laboratory data on serum potassium from the U.S. placebo-controlled trials are shown in the following table:
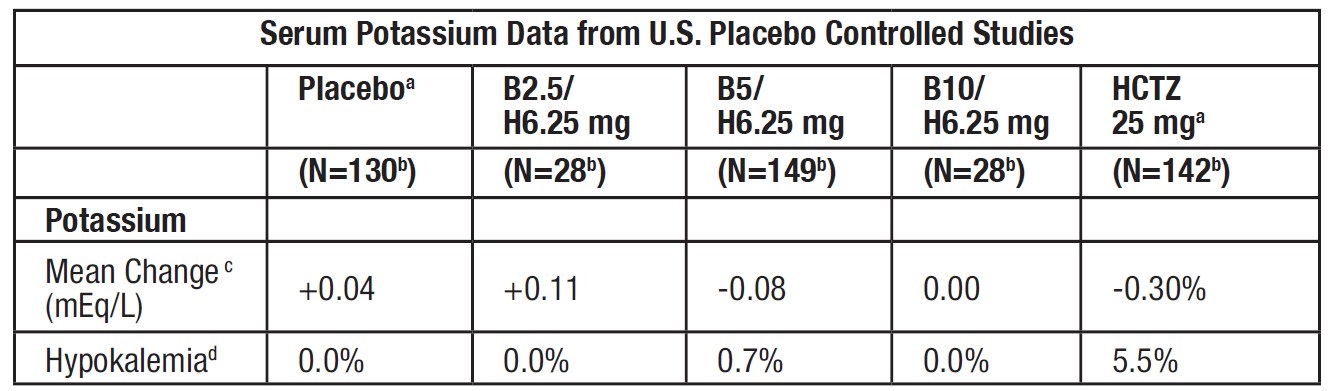
a) Combined across studies.
b) Patients with normal serum potassium at baseline.
c) Mean change from baseline at Week 4.
d) Percentage of patients with abnormality at Week 4.
Treatment with both beta blockers and thiazide diuretics is associated with increases in uric acid. However, the magnitude of the change in patients treated with B/H 6.25 mg was smaller than in patients treated with HCTZ 25 mg. Mean increases in serum triglycerides were observed in patients treated with bisoprolol fumarate and hydrochlorothiazide 6.25 mg. Total cholesterol was generally unaffected, but small decreases in HDL cholesterol were noted.
Other laboratory abnormalities that have been reported with the individual components are listed below.
Bisoprolol Fumarate
In clinical trials, the most frequently reported laboratory change was an increase in serum triglycerides, but this was not a consistent finding.
Sporadic liver test abnormalities have been reported. In the U.S. controlled trials experience with bisoprolol fumarate treatment for 4-12 weeks, the incidence of concomitant elevations in SGOT and SGPT from 1 to 2 times normal was 3.9%, compared to 2.5% for placebo. No patient had concomitant elevations greater than twice normal.
In the long-term, uncontrolled experience with bisoprolol fumarate treatment for 6-18 months, the incidence of one or more concomitant elevations in SGOT and SGPT from 1 to 2 times normal was 6.2%. The incidence of multiple occurrences was 1.9%. For concomitant elevations in SGOT and SGPT of greater than twice normal, the incidence was 1.5%. The incidence of multiple occurrences was 0.3%. In many cases these elevations were attributed to underlying disorders, or resolved during continued treatment with bisoprolol fumarate.
Other laboratory changes included small increases in uric acid, creatinine, BUN, serum potassium, glucose, and phosphorus and decreases in WBC and platelets. There have been occasional reports of eosinophilia. These were generally not of clinical importance and rarely resulted in discontinuation of bisoprolol fumarate.
As with other beta-blockers, ANA conversions have also been reported on bisoprolol fumarate. About 15% of patients in long-term studies converted to a positive titer, although about one-third of these patients subsequently reconverted to a negative titer while on continued therapy.
Hydrochlorothiazide
Hyperglycemia, glycosuria, hyperuricemia, hypokalemia and other electrolyte imbalances (see PRECAUTIONS), hyperlipidemia, hypercalcemia, leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia, and hemolytic anemia have been associated with HCTZ therapy.
Close -
OVERDOSAGEThere are limited data on overdose with bisoprolol fumarate and hydrochlorothiazide tablets. However, several cases of overdose with bisoprolol fumarate have been reported (maximum: 2000 mg) ...
There are limited data on overdose with bisoprolol fumarate and hydrochlorothiazide tablets. However, several cases of overdose with bisoprolol fumarate have been reported (maximum: 2000 mg). Bradycardia and/or hypotension were noted. Sympathomimetic agents were given in some cases, and all patients recovered.
The most frequently observed signs expected with overdosage of a beta-blocker are bradycardia and hypotension. Lethargy is also common, and with severe overdoses, delirium, coma, convulsions, and respiratory arrest have been reported to occur. Congestive heart failure, bronchospasm, and hypoglycemia may occur, particularly in patients with underlying conditions. With thiazide diuretics, acute intoxication is rare. The most prominent feature of overdose is acute loss of fluid and electrolytes. Signs and symptoms include cardiovascular (tachycardia, hypotension, shock), neuromuscular (weakness, confusion, dizziness, cramps of the calf muscles, paresthesia, fatigue, impairment of consciousness), gastrointestinal (nausea, vomiting, thirst), renal (polyuria, oliguria, or anuria [due to hemoconcentration]), and laboratory findings (hypokalemia, hyponatremia, hypochloremia, alkalosis, increased BUN [especially in patients with renal insufficiency]).
If overdosage of bisoprolol fumarate and hydrochlorothiazide tablets is suspected, therapy with bisoprolol fumarate and hydrochlorothiazide tablets should be discontinued and the patient observed closely. Treatment is symptomatic and supportive; there is no specific antidote. Limited data suggest bisoprolol fumarate is not dialyzable; similarly, there is no indication that hydrochlorothiazide is dialyzable. Suggested general measures include induction of emesis and/or gastric lavage, administration of activated charcoal, respiratory support, correction of fluid and electrolyte imbalance, and treatment of convulsions. Based on the expected pharmacologic actions and recommendations for other beta- blockers and hydrochlorothiazide, the following measures should be considered when clinically warranted:
Bradycardia
Administer IV atropine. If the response is inadequate, isoproterenol or another agent with positive chronotropic properties may be given cautiously. Under some circumstances, transvenous pacemaker insertion may be necessary.
Hypotension, Shock
The patient’s legs should be elevated. IV fluids should be administered and lost electrolytes (potassium, sodium) replaced. Intravenous glucagon may be useful. Vasopressors should be considered.
Heart Block (second or third degree)
Patients should be carefully monitored and treated with isoproterenol infusion or transvenous cardiac pacemaker insertion, as appropriate.
Congestive Heart Failure
Initiate conventional therapy (ie, digitalis, diuretics, vasodilating agents, inotropic agents).
Bronchospasm
Administer a bronchodilator such as isoproterenol and/or aminophylline.
Hypoglycemia
Administer IV glucose.
Surveillance Fluid and electrolyte balance (especially serum potassium) and renal function should be monitored until normalized.
Close -
DOSAGE & ADMINISTRATIONBisoprolol is an effective treatment of hypertension in once-daily doses of 2.5 to 40 mg, while hydrochlorothiazide is effective in doses of 12.5 to 50 mg. In clinical trials of ...
Bisoprolol is an effective treatment of hypertension in once-daily doses of 2.5 to 40 mg, while hydrochlorothiazide is effective in doses of 12.5 to 50 mg. In clinical trials of bisoprolol/hydrochlorothiazide combination therapy using bisoprolol doses of 2.5 to 20 mg and hydrochlorothiazide doses of 6.25 to 25 mg, the antihypertensive effects increased with increasing doses of either component.
The adverse effects (see WARNINGS) of bisoprolol are a mixture of dose-dependent phenomena (primarily bradycardia, diarrhea, asthenia, and fatigue) and dose-independent phenomena (eg, occasional rash); those of hydrochlorothiazide are a mixture of dose-dependent phenomena (primarily hypokalemia) and dose-independent phenomena (eg, possibly pancreatitis); the dose- dependent phenomena for each being much more common than the dose-independent phenomena. The latter consist of those few that are truly idiosyncratic in nature or those that occur with such low frequency that a dose relationship may be difficult to discern. Therapy with a combination of bisoprolol and hydrochlorothiazide will be associated with both sets of dose- independent adverse effects, and to minimize these, it may be appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy. On the other hand, regimens that combine low doses of bisoprolol and hydrochlorothiazide should produce minimal dose-dependent adverse effects, eg, bradycardia, diarrhea, asthenia and fatigue, and minimal dose-dependent adverse metabolic effects, ie, decreases in serum potassium (see CLINICAL PHARMACOLOGY).
Therapy Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with 2.5-20 mg bisoprolol daily may instead be given bisoprolol fumarate and hydrochlorothiazide tablets. Patients whose blood pressures are adequately controlled with 50 mg of hydrochlorothiazide daily, but who experience significant potassium loss with this regimen, may achieve similar blood pressure control without electrolyte disturbance if they are switched to bisoprolol fumarate and hydrochlorothiazide tablets.
Initial Therapy
Antihypertensive therapy may be initiated with the lowest dose of bisoprolol fumarate and hydrochlorothiazide tablets, one 2.5/6.25 mg tablet once daily. Subsequent titration (14 day intervals) may be carried out with bisoprolol fumarate and hydrochlorothiazide tablets up to the maximum recommended dose 20/12.5 mg (two 10/6.25 mg tablets) once daily, as appropriate.
Replacement Therapy
The combination may be substituted for the titrated individual components.
Cessation of Therapy
If withdrawal of bisoprolol fumarate and hydrochlorothiazide tablets therapy is planned, it should be achieved gradually over a period of about 2 weeks. Patients should be carefully observed.
Patients with Renal or Hepatic Impairment: As noted in the WARNINGS section, caution must be used in dosing/titrating patients with hepatic impairment or renal dysfunction. Since there is no indication that hydrochlorothiazide is dialyzable, and limited data suggest that bisoprolol is not dialyzable, drug replacement is not necessary in patients undergoing dialysis.
Geriatric Patients: Dosage adjustment on the basis of age is not usually necessary, unless there is also significant renal or hepatic dysfunction (see above and WARNINGS section).
Pediatric Patients: There is no pediatric experience with bisoprolol fumarate and hydrochlorothiazide tablets.
Close -
HOW SUPPLIEDBisoprolol fumarate and hydrochlorothiazide tablets, 2.5 mg/6.25 mg: Yellow, round, biconvex, film-coated, unscored tablets, debossed with “920” on one side and plain on the reverse side, supplied ...
Bisoprolol fumarate and hydrochlorothiazide tablets, 2.5 mg/6.25 mg:
Yellow, round, biconvex, film-coated, unscored tablets, debossed with “920” on one side and plain on the reverse side, supplied as follows:
Bottle of 30 Tablets
NDC 42799-920-30
Bottle of 100 Tablets
NDC 42799-920-01
Bottle of 500 Tablets
NDC 42799-920-02
Bisoprolol fumarate and hydrochlorothiazide tablets, 5 mg/6.25 mg:
Pink, round, biconvex, film-coated, unscored tablets, debossed with “921” on one side and plain on the reverse side, supplied as follows:
Bottle of 30 Tablets
NDC 42799-921-30
Bottle of 100 Tablets
NDC 42799-921-01
Bottle of 500 Tablets
NDC 42799-921-02
Bisoprolol fumarate and hydrochlorothiazide tablets, 10 mg/6.25 mg:
White to off-white, round, biconvex, film-coated, unscored tablets, debossed with “922” on one side and plain on the reverse side, supplied as follows:
Bottle of 30 Tablets
NDC 42799-922-30
Bottle of 100 Tablets
NDC 42799-922-01
Bottle of 500 Tablets
NDC 42799-922-02
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight container.
Edenbridge Pharmaceuticals, LLC
Close
Parsippany, New Jersey, 07054
Revised January 2024 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information
BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE bisoprolol fumarate and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42799-920 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISOPROLOL FUMARATE (UNII: UR59KN573L) (BISOPROLOL - UNII:Y41JS2NL6U) BISOPROLOL FUMARATE 2.5 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 6.25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color YELLOW Score no score Shape ROUND Size 7mm Flavor Imprint Code 920 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42799-920-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC:42799-920-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC:42799-920-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212678 04/30/2019 BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE bisoprolol fumarate and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42799-921 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISOPROLOL FUMARATE (UNII: UR59KN573L) (BISOPROLOL - UNII:Y41JS2NL6U) BISOPROLOL FUMARATE 5 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 6.25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) D&C RED NO. 30 (UNII: 2S42T2808B) Product Characteristics Color PINK Score no score Shape ROUND Size 7mm Flavor Imprint Code 921 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42799-921-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC:42799-921-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC:42799-921-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212678 04/30/2019 BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE bisoprolol fumarate and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42799-922 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISOPROLOL FUMARATE (UNII: UR59KN573L) (BISOPROLOL - UNII:Y41JS2NL6U) BISOPROLOL FUMARATE 10 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 6.25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code 922 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42799-922-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 2 NDC:42799-922-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 3 NDC:42799-922-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212678 04/30/2019
CloseLabeler - Edenbridge Pharmaceuticals LLC. (948715060)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE tablet, film coated
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 854908 | bisoprolol fumarate 10 MG / hydroCHLOROthiazide 6.25 MG Oral Tablet | PSN |
| 2 | 854908 | bisoprolol fumarate 10 MG / hydrochlorothiazide 6.25 MG Oral Tablet | SCD |
| 3 | 854908 | bisoprolol fumarate 10 MG / HCTZ 6.25 MG Oral Tablet | SY |
| 4 | 854916 | bisoprolol fumarate 2.5 MG / hydroCHLOROthiazide 6.25 MG Oral Tablet | PSN |
| 5 | 854916 | bisoprolol fumarate 2.5 MG / hydrochlorothiazide 6.25 MG Oral Tablet | SCD |
| 6 | 854916 | bisoprolol fumarate 2.5 MG / HCTZ 6.25 MG Oral Tablet | SY |
| 7 | 854919 | bisoprolol fumarate 5 MG / hydroCHLOROthiazide 6.25 MG Oral Tablet | PSN |
| 8 | 854919 | bisoprolol fumarate 5 MG / hydrochlorothiazide 6.25 MG Oral Tablet | SCD |
| 9 | 854919 | bisoprolol fumarate 5 MG / HCTZ 6.25 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=295532b5-65c8-40c3-8d27-cfd334d12802
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 42799-920-01 |
| 2 | 42799-920-02 |
| 3 | 42799-920-30 |
| 4 | 42799-921-01 |
| 5 | 42799-921-02 |
| 6 | 42799-921-30 |
| 7 | 42799-922-01 |
| 8 | 42799-922-02 |
| 9 | 42799-922-30 |

















