Label: ADEFOVIR DIPIVOXIL tablet
- NDC Code(s): 42794-003-08
- Packager: Sigmapharm Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADEFOVIR DIPIVOXIL TABLETS safely and effectively. See full prescribing information for ADEFOVIR DIPIVOXIL TABLETS. ADEFOVIR ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS, NEPHROTOXICITY, HIV RESISTANCE, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS
Severe acute exacerbations of hepatitis have been reported in patients who have discontinued anti-Hepatitis B therapy including Adefovir Dipivoxil Tablets. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-Hepatitis B therapy. If appropriate, resumption of anti-Hepatitis B therapy may be warranted [See Warnings and Precautions (5.1)] .
In patients at risk of or having underlying renal dysfunction, chronic administration of Adefovir Dipivoxil Tablets may result in nephrotoxicity. These patients should be monitored closely for renal function and may require dose adjustment [See Warnings and Precautions (5.2) and Dosage and Administration (2.2)] .
HIV resistance may emerge in chronic hepatitis B patients with unrecognized or untreated Human Immunodeficiency Virus (HIV) infection treated with antihepatitis B therapies, such as therapy with Adefovir Dipivoxil Tablets, that may have activity against HIV [See Warnings and Precautions (5.3)] .
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals [See Warnings and Precautions (5.4)] .
Close -
1 INDICATIONS AND USAGEAdefovir Dipivoxil Tablets are indicated for the treatment of chronic hepatitis B in patients 12 years of age and older with evidence of active viral replication and either evidence of persistent ...
-
2 DOSAGE AND ADMINISTRATION2.1 Chronic Hepatitis B - The recommended dose of Adefovir Dipivoxil Tablets in chronic hepatitis B patients for patients 12 years of age and older with adequate renal function is 10 mg, once ...
-
3 DOSAGE FORMS AND STRENGTHSAdefovir Dipivoxil is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white, round, flat faced beveled edged tablets, debossed Σ 3 on one side and plain on ...
-
4 CONTRAINDICATIONSAdefovir Dipivoxil Tablets are contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the product.
-
5 WARNINGS AND PRECAUTIONS5.1 Exacerbation of Hepatitis after Discontinuation of Treatment - Severe acute exacerbation of hepatitis has been reported in patients who have discontinued anti-hepatitis B therapy ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: Severe acute exacerbations of Hepatitis - [See - Boxed Warning ...
-
7 DRUG INTERACTIONSSince adefovir is eliminated by the kidney, coadministration of Adefovir Dipivoxil Tablets with drugs that reduce renal function or compete for active tubular secretion may increase serum ...
-
8 USE IN SPECIFIC POPULATION8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Adefovir Dipivoxil Tablets during pregnancy ...
-
10 OVERDOSAGEDoses of adefovir dipivoxil 500 mg daily for 2 weeks and 250 mg daily for 12 weeks have been associated with gastrointestinal side effects. If overdose occurs the patient must be monitored for ...
-
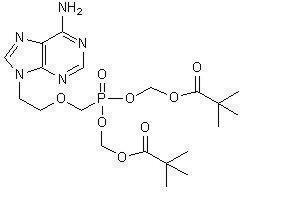
11 DESCRIPTIONAdefovir dipivoxil is a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The chemical name of adefovir dipivoxil is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Adefovir is an antiviral drug - [See - Microbiology (12.4)]. 12.3 Pharmacokinetics - Adult Subjects - The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term oral carcinogenicity studies of adefovir dipivoxil in mice and rats were carried out at exposures up to approximately ...
-
14 CLINICAL STUDIES14.1 Studies 437 and 438 (Pivotal Studies) HBeAg-Positive Chronic Hepatitis B - Study 437 was a randomized, double-blind, placebo-controlled, three-arm-study in patients with ...
-
16 HOW SUPPLIED / STORAGE AND HANDLINGAdefovir Dipivoxil is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white, round, flat faced beveled edged tablets, debossed ∑3 on one side and plain on ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients of the potential risks and benefits of Adefovir Dipivoxil Tablets and of alternative modes ...
-
PATIENT PACKAGE INSERTFDA-Approved Patient Labeling - PATIENT INFORMATION - ADEFOVIR DIPIVOXIL (a-DEF-oh-vir dip-ih-VOX-il) TABLETS - Read this Patient Information before you start taking Adefovir Dipivoxil Tablets ...
-
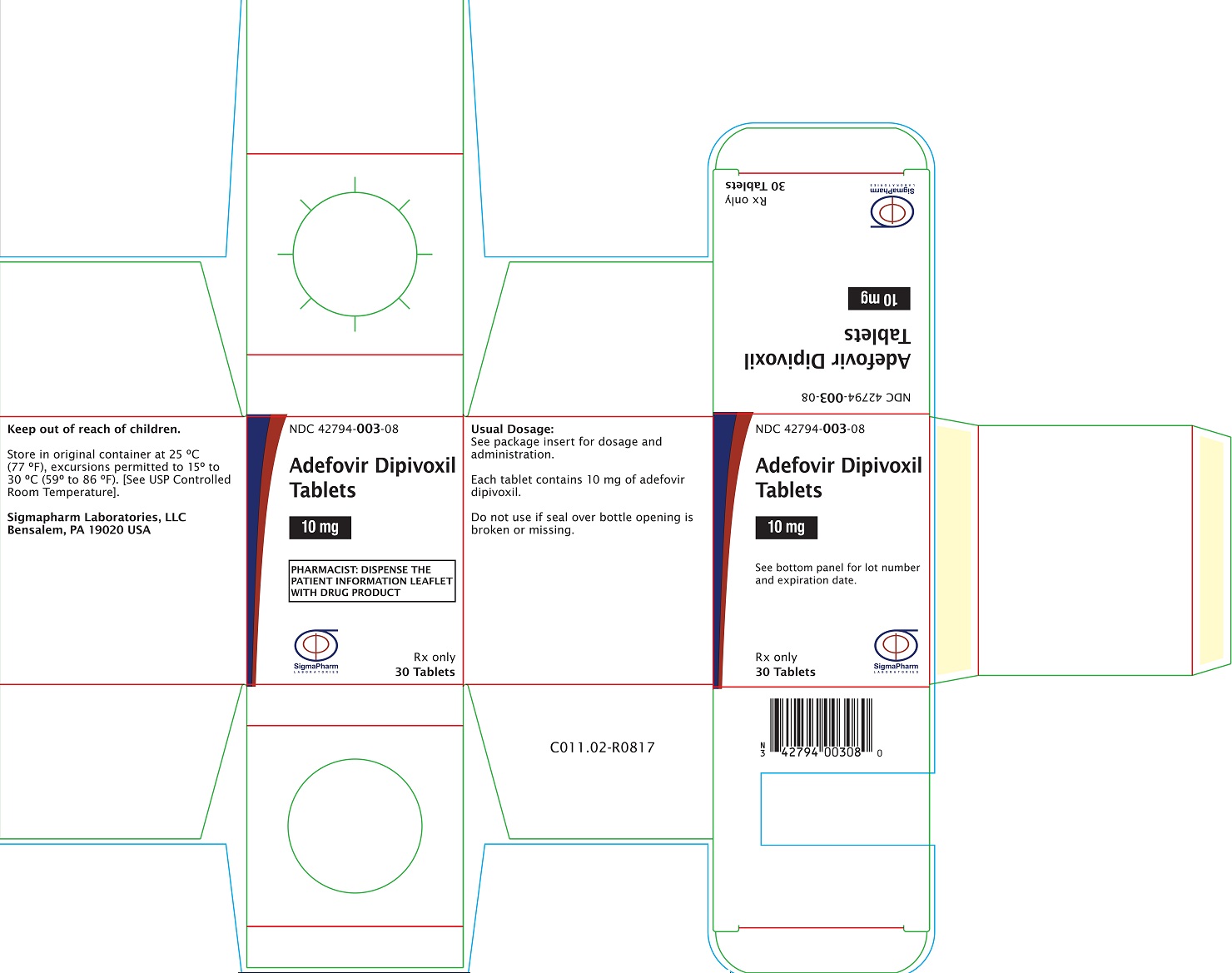
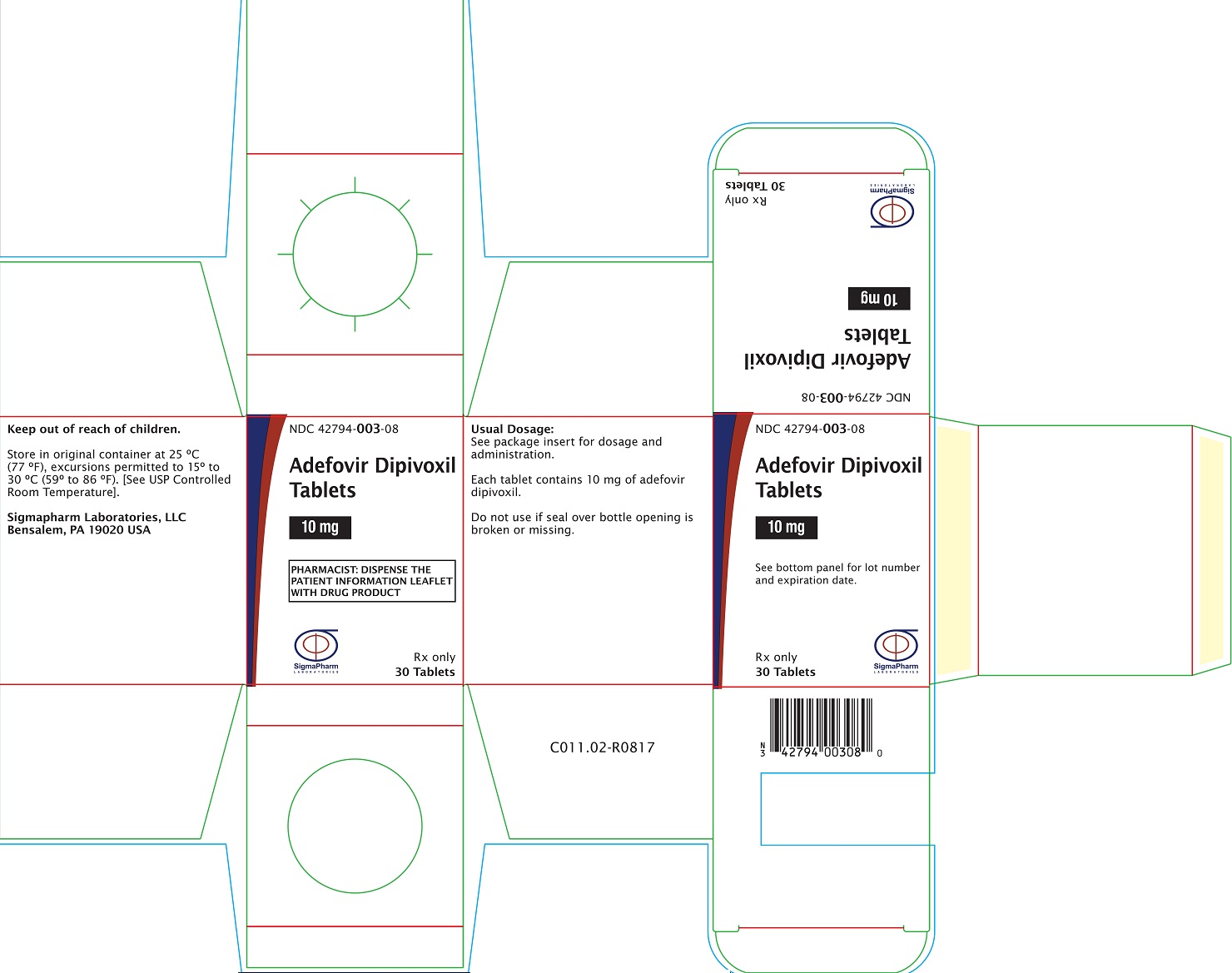
PRINCIPAL DISPLAY PANEL- Adefovir Dipivoxil 10 mg Bottle Label
Sigmapharm Laboratories, LLC - NDC 42794-003-08 - Adefovir Dipivoxil Tablets - 10 mg - PHARMACIST: DISPENSE THE PATIENT INFORMATION LEAFLET WITH DRUG PRODUCT - Rx Only - 30 Tablets
-
PRINCIPAL DISPLAY PANEL- Adefovir Dipivoxil 10 mg Carton Label
Sigmapharm Laboratories, LLC - NDC 42794- 003-08 - Adefovir Dipivoxil Tablets - 10 mg - PHARMACIST: DISPENSE THE PATIENT INFORMATION LEAFLET WITH DRUG ...
-
INGREDIENTS AND APPEARANCEProduct Information