Label: SODIUM SULFACETAMIDE 9.8%, SULFUR 4.8%- sodium sulfacetamide, sulfur solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 42582-801-11 - Packager: Bi-Coastal Pharma International LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
-
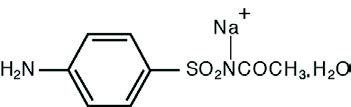
DESCRIPTIONDESCRIPTION: Each gram of Sodium Sulfacetamide and Sulfur (sodium sulfacetamide 9.8% w/w and sulfur 4.8% w/w) contains 98 mg of sodium sulfacetamide and 48 mg of colloidal sulfur in a vehicle ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to ...
-
INDICATIONS & USAGEINDICATIONS: Sodium Sulfacetamide 9.8% and Sulfur 4.8% Cleanser is indicated for use in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
-
CONTRAINDICATIONSCONTRAINDICATIONS: Sodium Sulfacetamide 9.8% and Sulfur 4.8% Cleanser is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this ...
-
WARNINGSWARNINGS: Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to ...
-
PRECAUTIONSPRECAUTIONS: FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. General: If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be ...
-
ADVERSE REACTIONSADVERSE REACTIONS: Although rare, sodium sulfacetamide may cause local irritation. Call your doctor for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONS, contact the FDA at ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Wash affected areas with Sodium Sulfacetamide 9.8% and Sulfur 4.8% Cleanser one to two times daily, or as directed by your physician. Avoid contact with eyes or mucous ...
-
HOW SUPPLIEDHOW SUPPLIED: Sodium Sulfacetamide 9.8% and Sulfur 4.8% Cleanser is supplied in an 8 oz. (227 g) bottle, NDC 42582-801-11. Store at 25°C (77°F); excursions permitted to between 15°C to 30°C (59°F ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information