Label: IBUPROFEN tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 42582-111-10, 42582-111-18, 42582-112-10, 42582-112-18, view more - Packager: Bi-Coastal Pharma International LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (see WARNINGS and PRECAUTIONS).
- Ibuprofen tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see CONTRAINDICATIONS and WARNINGS).
Gastrointestinal Risk
- NSAIDS cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
-
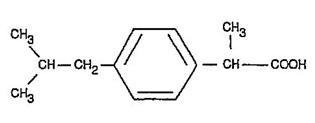
DESCRIPTIONIbuprofen tablets contain the active ingredient ibuprofen, which is (±) - 2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77° C and is very ...
-
CLINICAL PHARMACOLOGYIbuprofen tablets contain ibuprofen which possesses analgesic and antipyretic activities. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to ...
-
INDICATIONS AND USAGECarefully consider the potential benefits and risks of ibuprofen tablets and other treatment options before deciding to use ibuprofen tablets. Use the lowest effective dose for the shortest ...
-
CONTRAINDICATIONSIbuprofen tablets are contraindicated in patients with known hypersensitivity to Ibuprofen. Ibuprofen tablets should not be given to patients who have experienced asthma, urticaria, or ...
-
WARNINGSCardiovascular Effects - Cardiovascular Thrombotic Events - Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of ...
-
PRECAUTIONSGeneral Precautions - Ibuprofen tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to ...
-
ADVERSE REACTIONSThe most frequent type of adverse reaction occurring with ibuprofen tablets is gastrointestinal . In controlled clinical trials the percentage of patients reporting one or more gastrointestinal ...
-
OVERDOSAGEApproximately 1½ hours after the reported ingestion of from 7 to 10 ibuprofen tablets (400 mg), a 19-month old child weighing 12 kg was seen in the hospital emergency room, apneic and cyanotic ...
-
DOSAGE AND ADMINISTRATIONCarefully consider the potential benefits and risks of ibuprofen tablets and other treatment options before deciding to use ibuprofen tablets. Use the lowest effective dose for the shortest ...
-
HOW SUPPLIEDIbuprofen tablets are available in the following strengths: 400 mg (white to off white, round, biconvex, film coated tablets debossed with ‘121’ on one side and plain on other side) Bottles of ...
-
SPL MedguideMedication Guide for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) What is the most important information I should know about medicines called Nonsteroidal Anti-Inflammatory Drugs ...
-
PRINCIPAL DISPLAY PANELNDC 42582-111-10 - Bi-Coastal Pharma Inertnational, LLC - IBUPROFEN - TABLETS, UPS - 400 mg - Parmacist: Dispence the Medicatlone Guide provided separatelu to each patient - Rx ONLY 100 Tablets - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information