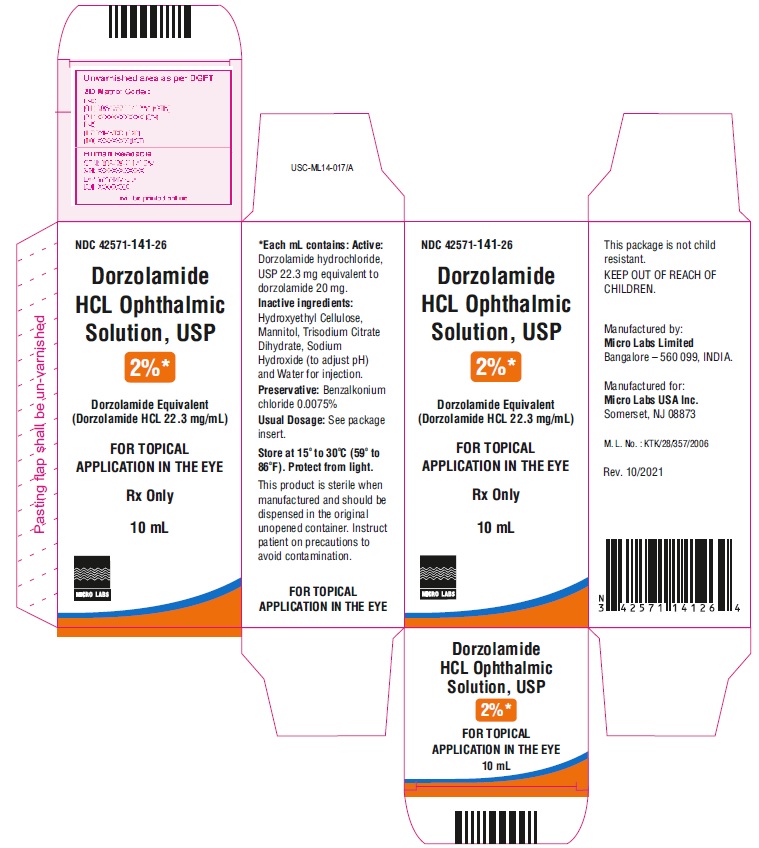
Label: DORZOLAMIDE HYDROCHLORIDE OPHTHALMIC SOLUTION- dorzolamide hydrochloride solution/ drops
- NDC Code(s): 42571-141-26
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DORZOLAMIDE HYDROCHLORIDE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for DORZOLAMIDE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDorzolamide hydrochloride ophthalmic solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
-
2 DOSAGE AND ADMINISTRATIONThe dose is one drop of dorzolamide hydrochloride ophthalmic solution in the affected eye(s) three times daily. Dorzolamide hydrochloride ophthalmic solution may be used concomitantly with other ...
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing dorzolamide 2% (20 mg/mL) equivalent to 22.3 mg/mL of dorzolamide hydrochloride.
-
4 CONTRAINDICATIONSDorzolamide hydrochloride ophthalmic solution is contraindicated in patients who are hypersensitive to any component of this product - [see - Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Sulfonamide Hypersensitivity - Dorzolamide hydrochloride ophthalmic solution contains dorzolamide, a sulfonamide; and although administered topically, it is absorbed systemically. Therefore ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Oral Carbonic Anhydrase Inhibitors - There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women with dorzolamide hydrochloride ophthalmic solution. Dorzolamide caused fetal vertebral ...
-
10 OVERDOSAGEElectrolyte imbalance, development of an acidotic state, and possible central nervous system effects may occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be ...
-
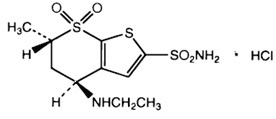
11 DESCRIPTIONDorzolamide hydrochloride ophthalmic solution, USP is a carbonic anhydrase inhibitor formulated for topical ophthalmic use. Dorzolamide hydrochloride USP is described chemically as: (4 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Carbonic anhydrase (CA) is an enzyme found in many tissues of the body including the eye. It catalyzes the reversible reaction involving the hydration of carbon dioxide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year study of dorzolamide hydrochloride administered orally to male and female Sprague- Dawley rats, urinary bladder ...
-
14 CLINICAL STUDIESThe efficacy of dorzolamide hydrochloride ophthalmic solution was demonstrated in clinical studies in the treatment of elevated intraocular pressure in patients with glaucoma or ocular ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDorzolamide hydrochloride ophthalmic solution, USP 2% is supplied in an LDPE white opaque cylindrical shape, screw type neck dispenser bottle closed with a LDPE white opaque cone shaped open ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Sulfonamide Reactions - Dorzolamide hydrochloride ophthalmic solution is a sulfonamide and although ...
-
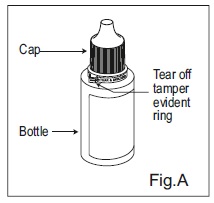
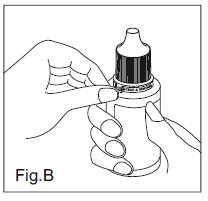
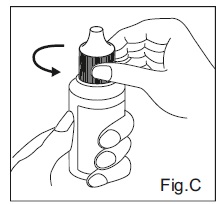
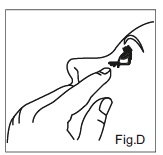
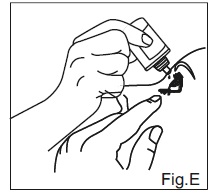
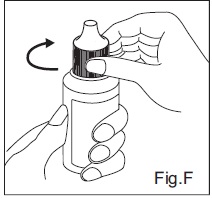
INSTRUCTIONS FOR USEDorzolamide Hydrochloride (dor zoe’ la mide hye” droe klor’ ide) Ophthalmic Solution USP, 2% 1. Before using the medication for the first time, be sure the tamper evident ring between the bottle ...
-
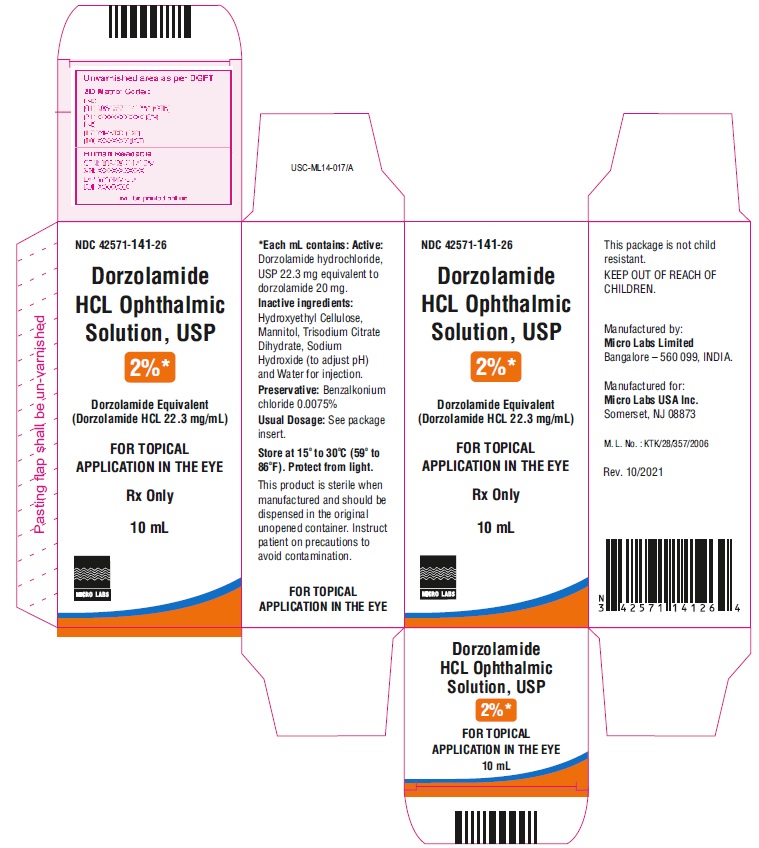
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 42571-141-26 - Rx Only - Dorzolamide HCl Ophthalmic - Solution, USP 2%* Dorzolamide Equivalent - (Dorzolamide HCl 22.3 mg/mL) FOR OPHTHALMIC APPLICATION - IN ...
-
INGREDIENTS AND APPEARANCEProduct Information