Label: ASPIRIN AND EXTENDED-RELEASE DIPYRIDAMOLE capsule, extended release
- NDC Code(s): 42571-274-60
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ASPIRIN and EXTENDED-RELEASE DIPYRIDAMOLE CAPSULES safely and effectively. See full prescribing information for ASPIRIN and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAspirin and extended-release dipyridamole capsule is indicated to reduce the risk of stroke in patients who have had transient ischemia of the brain or completed ischemic stroke due to ...
-
2 DOSAGE AND ADMINISTRATIONAspirin and extended-release dipyridamole capsule is not interchangeable with the individual components of aspirin and dipyridamole tablets. The recommended dose of aspirin and ...
-
3 DOSAGE FORMS AND STRENGTHSAspirin and extended-release Dipyridamole 25 mg/200 mg capsules are available as a hard gelatin capsule, with red opaque colored cap and ivory opaque colored body, size ‘0 Xel’ hard gelatin ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Aspirin and extended-release dipyridamole capsule is contraindicated in patients with known hypersensitivity to any of the product components. 4.2 Allergy - Aspirin is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Bleeding - Aspirin and extended-release dipyridamole increases the risk of bleeding. Risk factors for bleeding include the use of other drugs that increase the risk of bleeding (e.g. ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: Hypersensitivity [ see Contraindications - (4.1)] Allergy ...
-
7 DRUG INTERACTIONS7.1 Drug Interaction Study Information Obtained From Literature - Adenosinergic agents (e.g. adenosine, regadenoson) Dipyridamole has been reported to increase the plasma levels and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published studies and postmarketing experience with aspirin and extended-release dipyridamole capsules use during pregnancy have not ...
-
10 OVERDOSAGEBecause of the dose ratio of dipyridamole to aspirin, overdosage of aspirin and extended-release dipyridamole capsule is likely to be dominated by signs and symptoms of dipyridamole overdose. In ...
-
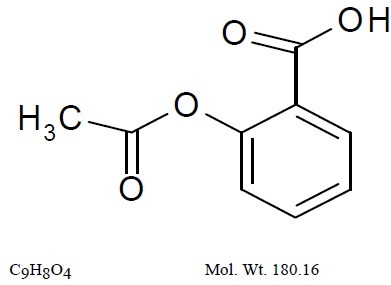
11 DESCRIPTIONAspirin and extended-release dipyridamole capsule is a combination antiplatelet agent intended for oral administration. Each hard gelatin capsule contains 200 mg dipyridamole in an ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The antithrombotic action of aspirin and extended-release dipyridamole capsule is the result of the additive antiplatelet effects of dipyridamole and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In studies in which dipyridamole was administered in the feed to mice (up to 111 weeks in males and females) and rats (up to 128 weeks ...
-
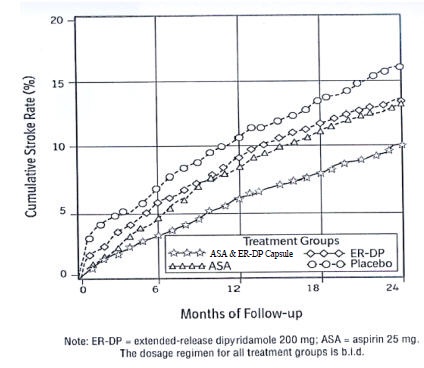
14 CLINICAL STUDIESESPS2 (European Stroke Prevention Study-2) was a double-blind, placebo-controlled, 24-month study in which 6602 patients over the age of 18 years had an ischemic stroke (76%) or transient ischemic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAspirin and extended-release Dipyridamole 25 mg/200 mg capsules are available as a hard gelatin capsule, with red opaque colored cap and ivory opaque colored body, size ‘0 Xel’ hard gelatin ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Risk of Bleeding - Inform patients that as with other antiplatelet agents, there is a general risk of ...
-
Patient InformationAspirin (AS-pir-in) and extended-release dipyridamole (dye-pir-id-a-mole) Capsules - Read this Patient Information before you start taking aspirin and extended-release dipyridamole capsule ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 42571-274-60 - Aspirin and - Extended-Release - Dipyridamole Capsules - 25 mg/200 mg - Unit-of-use Container - Rx only ...
-
INGREDIENTS AND APPEARANCEProduct Information