Label: HYDROCORTISONE ACETATE suppository
- NDC Code(s): 42494-342-12
- Packager: Cameron Pharmaceuticals, Limited Liability Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
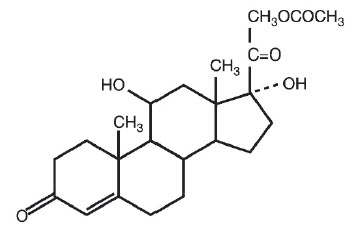
DESCRIPTIONHydrocortisone acetate is a corticosteroid designed chemically as pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β) with the following structural formula: Each suppository for ...
-
CLINICAL PHARMACOLOGYIn normal subjects, about 26% of hydrocortisone acetate is absorbed when the hydrocortisone acetate suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across ...
-
INDICATIONS AND USAGEHydrocortisone acetate suppositories are indicated for use in inflamed hemorrhoids, post-irradiation (factitial) proctitis, as an adjunct in the treatment of chronic ulcerative colitis, cryptitis ...
-
CONTRAINDICATIONSHydrocortisone acetate suppositories are contraindicated in those patients having a history of hypersensitivity to hydrocortisone acetate or any of the components.
-
PRECAUTIONSDo not use hydrocortisone acetate suppositories unless adequate proctologic examination is made. If irritation develops, the product should be discontinued and appropriate therapy instituted. In ...
-
ADVERSE REACTIONSThe following local adverse reactions have been reported with hydrocortisone acetate suppositories; burning, itching, irritation, dryness, folliculitis, hypopigmentation, allergic contact ...
-
DRUG ABUSE AND DEPENDENCEDrug abuse and dependence have not been reported in patients treated with hydrocortisone acetate suppositories.
-
OVERDOSAGEIf signs and symptoms of systemic overdosage occur, discontinue use.
-
DOSAGE AND ADMINISTRATIONFOR RECTAL ADMINISTRATION.Detach one suppository from strip of suppositories. Hold suppository upright and carefully separate tabs at top opening and pull downward from the pointed end to expose ...
-
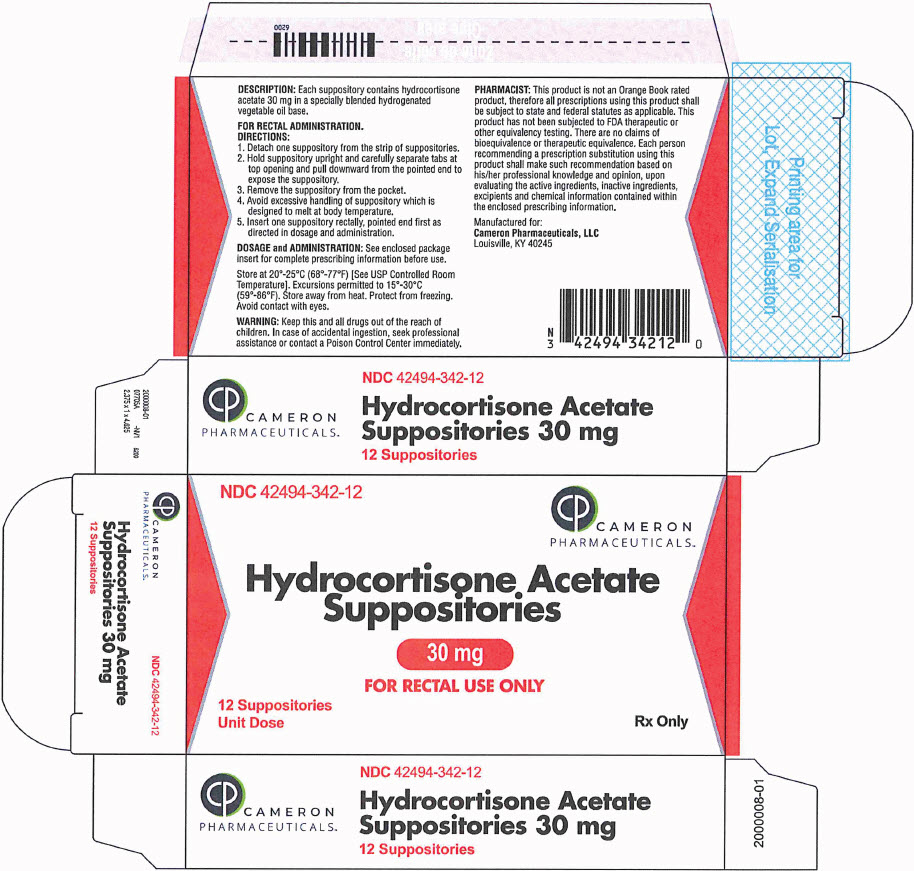
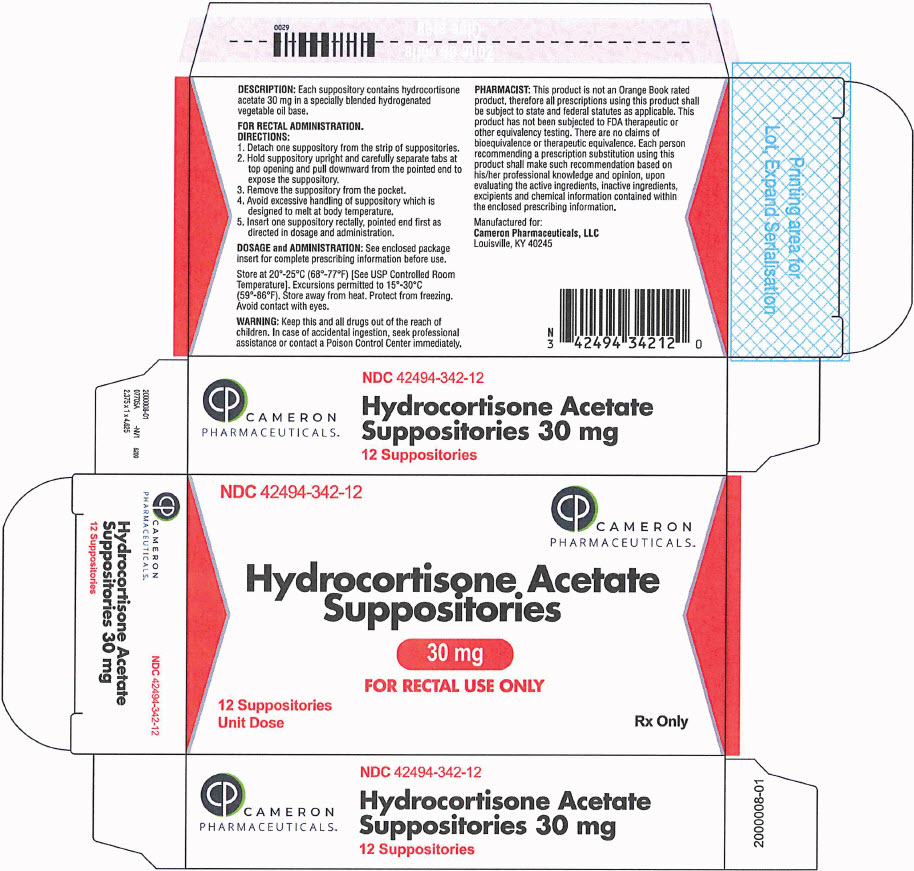
HOW SUPPLIEDHydrocortisone acetate suppositories 30mg are off-white, smooth surfaced and bullet shaped with one pointed end. Box of 12 suppositories, NDC 42494-342-12 - STORAGE - Store at 20°-25°C (68°-77°F ...
-
PHARMACISTThis product is not an Orange Book rated product, therefore all prescriptions using this product shall be subject to state and federal statutes as applicable. This product has not been subjected ...
-
SPL UNCLASSIFIED SECTIONRx Only - Manufacturing for: Cameron Pharmaceuticals, LLC - Louisville, KY 40245 - Rev. 08/21 - Hydrocortisone Acetate Suppositories 30mg - FOR RECTAL USE ONLY - 12 Suppositories - Unit ...
-
PRINCIPAL DISPLAY PANEL - 30 mg Suppository Blister Pack BoxNDC 42494-342-12 - CAMERON - PHARMACEUTICALS - ™ Hydrocortisone Acetate - Suppositories - 30 mg - FOR RECTAL USE ONLY - 12 Suppositories - Unit Dose - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information