Label: METFORMIN HYDROCHLORIDE tablet, extended release
- NDC Code(s): 42385-977-01, 42385-977-05, 42385-977-11, 42385-977-18, view more
- Packager: Laurus Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METFORMIN HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration (2.3), (2.7),Contraindications (4), Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin hydrochloride extended-release tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEMetformin hydrochloride extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Dosage - Metformin Hydrochloride Extended-Release Tablets - Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew. The recommended starting ...
-
3 DOSAGE FORMS AND STRENGTHSMetformin hydrochloride extended-release tablets, USP are available as: Metformin hydrochloride extended-release tablets, USP 500 mg are White to off-white color, capsule shaped, biconvex ...
-
4 CONTRAINDICATIONSMetformin hydrochloride extended-release tablets are contraindicated in patients with: Severe renal impairment (eGFR below 30 mL/min/1.73 m2) [see Warnings and Precautions (5.1)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Lactic Acidosis - There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific ...
-
6 ADVERSE REACTIONSThe following adverse reactions are also discussed elsewhere in the labeling: Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)] Vitamin B12 Deficiency [see Warnings and ...
-
7 DRUG INTERACTIONSTable 3 presents clinically significant drug interactions with metformin hydrochloride extended-release tablets. Table 3: Clinically Significant Drug Interactions with Metformin ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited data with metformin hydrochloride extended-release tablets in pregnant women are not sufficient to determine a drug-associated risk for major birth ...
-
10 OVERDOSAGEOverdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with ...
-
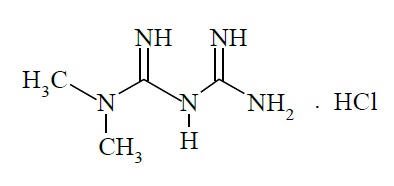
11 DESCRIPTIONMetformin hydrochloride extended-release tablets, USP contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at ...
-
14 CLINICAL STUDIES14.2 Metformin Hydrochloride Extended-Release Tablets - A 24-week, double-blind, placebo-controlled study of metformin hydrochloride extended-release tablets, taken once daily with the evening ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Metformin Hydrochloride Extended-Release Tablets, USP are supplied in the following strengths and package configurations: Metformin Hydrochloride Extended-Release Tablets ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Lactic Acidosis: Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to ...
-
PATIENT INFORMATIONMetformin Hydrochloride Extended-Release Tablets - [met-FOR-min HYE-droe-KLOR-ide] Read the Patient Information that comes with metformin hydrochloride extended-release tablets before you ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 500 mg - Container Label (30's count)NDC 42385-977-30 - Metformin Hydrochloride - Extended-Release - Tablets, USP - 500 mg - 30 Tablets Rx Only - LAURUS Labs
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 500 mg - Blister (1 x 10's count)Rx Only NDC 42385-977-17 - Metformin Hydrochloride - Extended-Release Tablets, USP - 500 mg
-
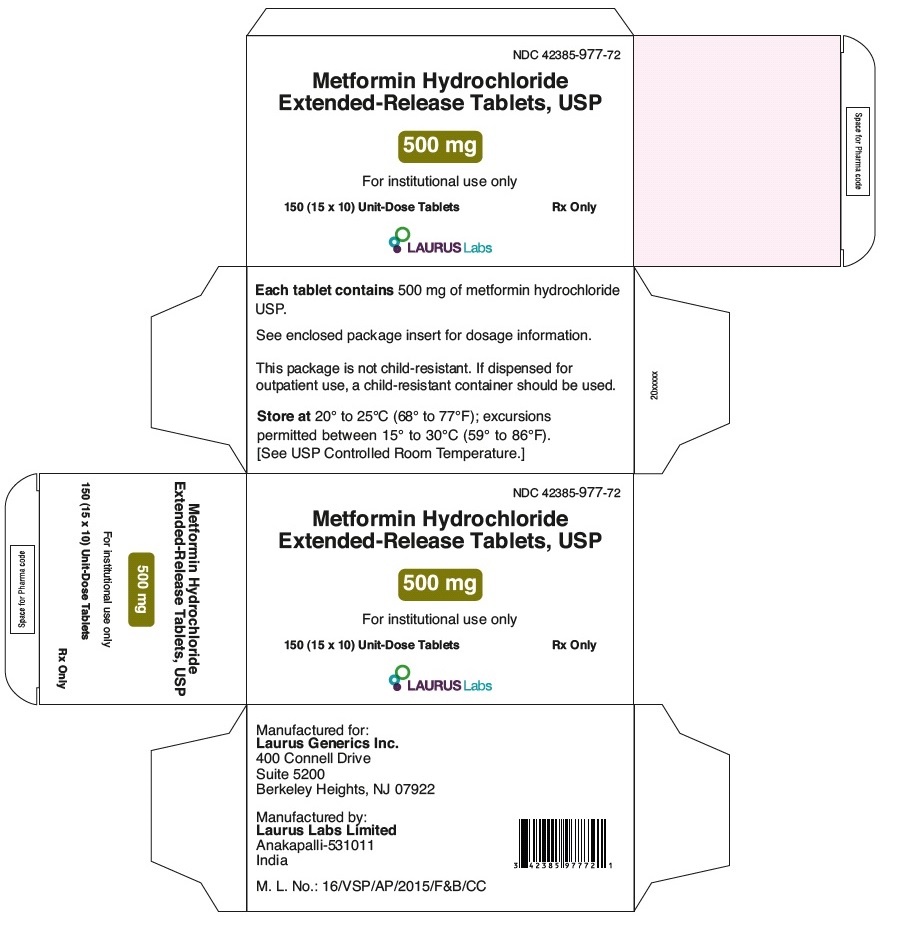
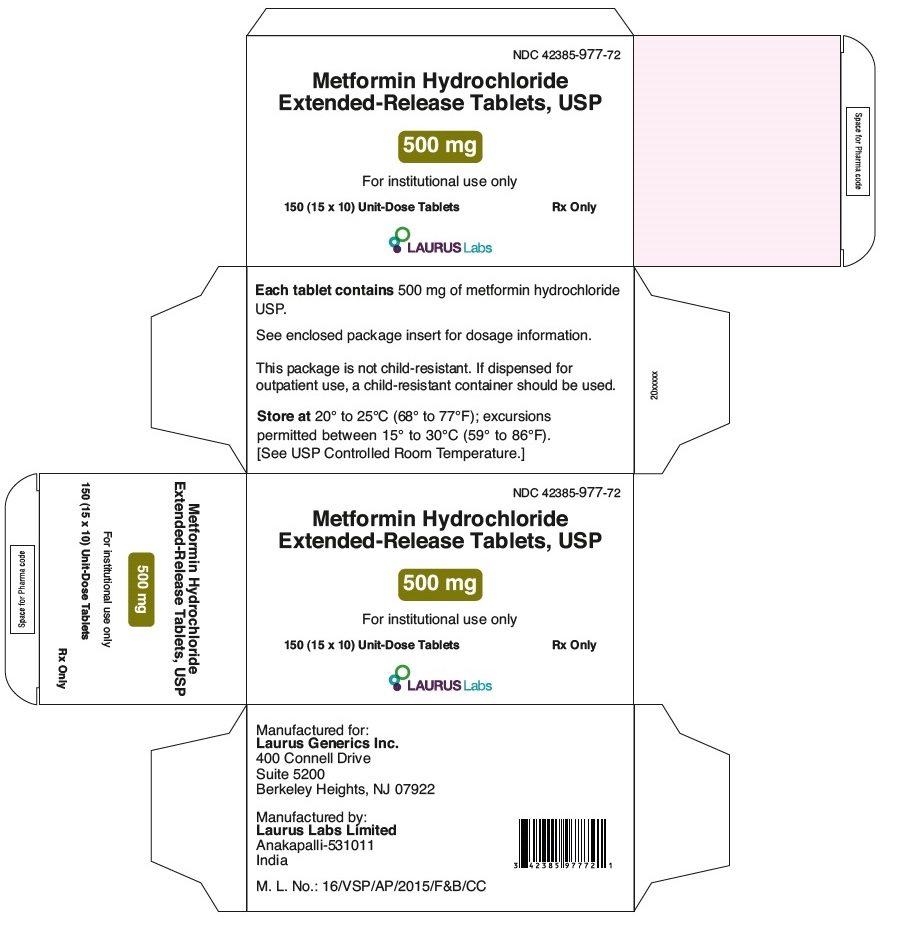
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 500 mg - Blister Carton - 150 (15 x 10) Unit-Dose TabletsNDC 42385-977-72 - Metformin Hydrochloride - Extended-Release Tablets, USP - 500 mg - For in-institution use only - 150 (15 x 10) Unit-Dose Tablets Rx Only - LAURUS Labs
-
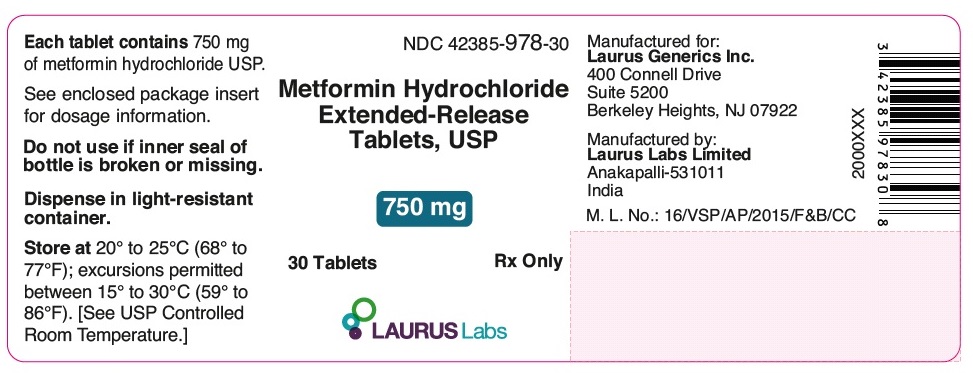
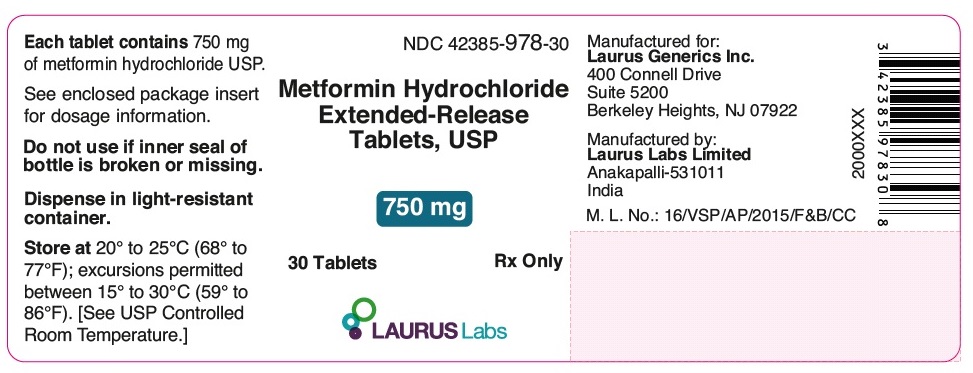
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 750 mg - Container Label (30's count)NDC 42385-978-30 - Metformin Hydrochloride - Extended-Release - Tablets, USP - 750 mg - 30 Tablets Rx Only - LAURUS Labs
-
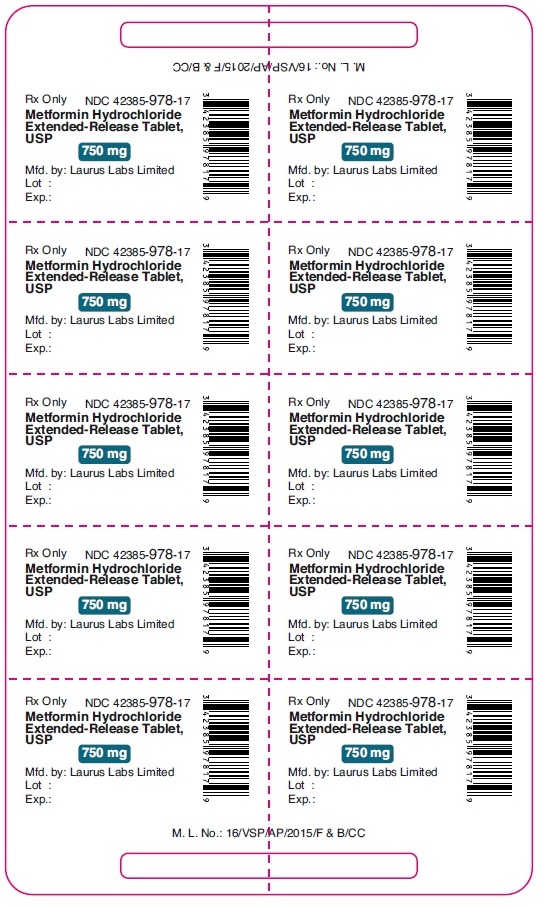
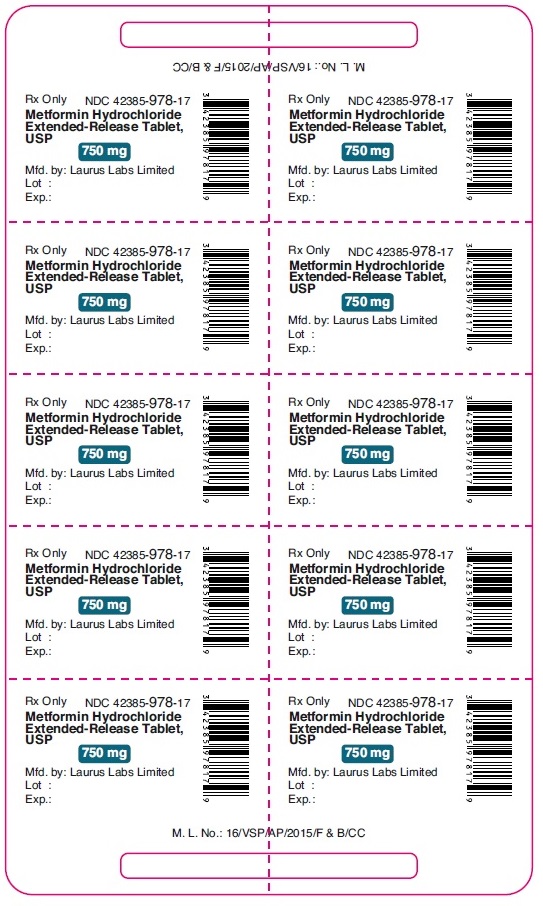
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 750 mg - Blister (1 x 10's count)Rx Only NDC 42385-978-17 - Metformin Hydrochloride - Extended-Release Tablet, USP - 750 mg
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 750 mg - Blister Carton - 150 (15 x 10) Unit-Dose TabletsNDC 42385-978-72 - Metformin Hydrochloride - Extended-Release Tablets, USP - 750 mg - 150 (15 x 10) Unit-Dose Tablets Rx Only - LAURUS Labs
-
INGREDIENTS AND APPEARANCEProduct Information