Label: ATORVASTATIN CALCIUM tablet, film coated
- NDC Code(s): 42385-940-01, 42385-940-05, 42385-940-10, 42385-940-11, view more
- Packager: Laurus Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ATORVASTATIN CALCIUM TABLETS safely and effectively. See full prescribing information for ATORVASTATIN CALCIUM TABLETS. ATORVASTATIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAtorvastatin calcium tablets are indicated: • To reduce the risk of: o Myocardial infarction (MI), stroke, revascularization procedures, and angina in adults with multiple risk factors for ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage Information - • Take atorvastatin calcium tablets orally once daily at any time of the day, with or without food. • Assess LDL-C when clinically appropriate, as early as 4 ...
-
3 DOSAGE FORMS AND STRENGTHSAtorvastatin calcium tablets, USP: 10 mg atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with “LA37” on one side and plain on the other side. 20 mg of ...
-
4 CONTRAINDICATIONS• Acute liver failure or decompensated cirrhosis [see Warnings and Precautions (5.3)] • Hypersensitivity to atorvastatin or any excipients in atorvastatin calcium tablets. Hypersensitivity ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myopathy and Rhabdomyolysis - Atorvastatin may cause myopathy (muscle pain, tenderness, or weakness associated with elevated creatine kinase [CK]) and rhabdomyolysis. Acute kidney injury ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described below and elsewhere in the labeling: • Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)] • Immune-Mediated Necrotizing ...
-
7 DRUG INTERACTIONS7.1 Drug Interactions that may Increase the Risk of Myopathy and Rhabdomyolysis with Atorvastatin - Atorvastatin is a substrate of CYP3A4 and transporters (e.g., OATP1B1/1B3, P-gp, or BCRP) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Discontinue atorvastatin when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient. Atorvastatin decreases ...
-
10 OVERDOSAGENo specific antidotes for atorvastatin are known. Contact Poison Control (1-800-222-1222) for latest recommendations. Due to extensive drug binding to plasma proteins, hemodialysis is not expected ...
-
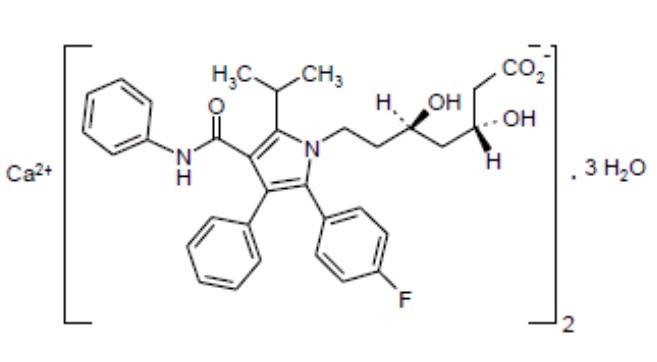
11 DESCRIPTIONAtorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Atorvastatin calcium is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year carcinogenicity study in rats at dose levels of 10, 30, and 100 mg/kg/day, 2 rare tumors were found in muscle in ...
-
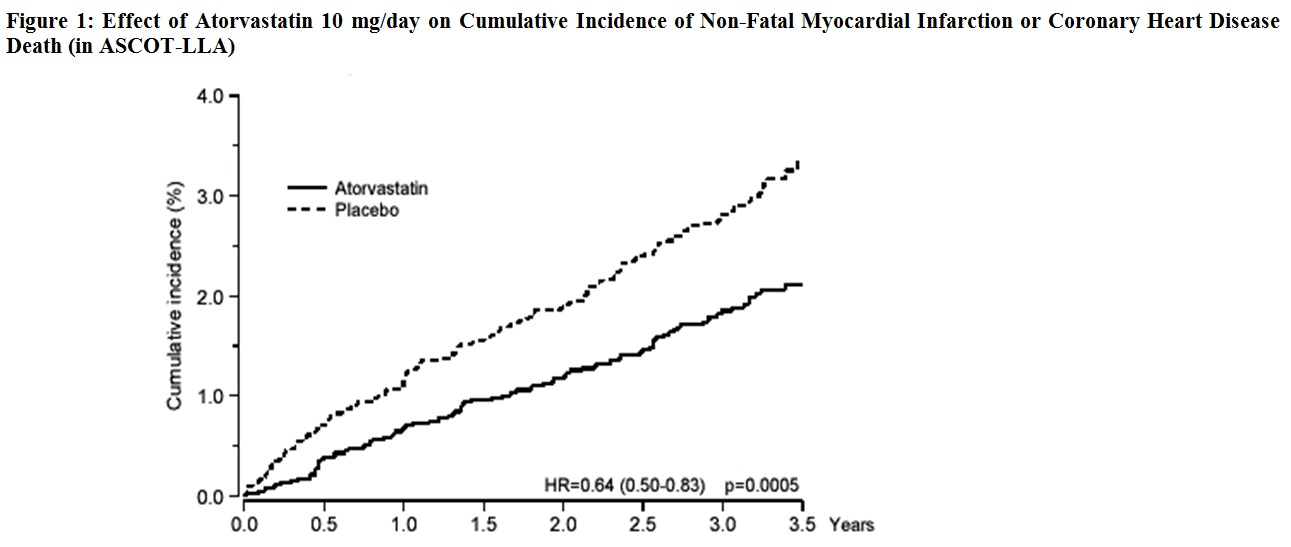
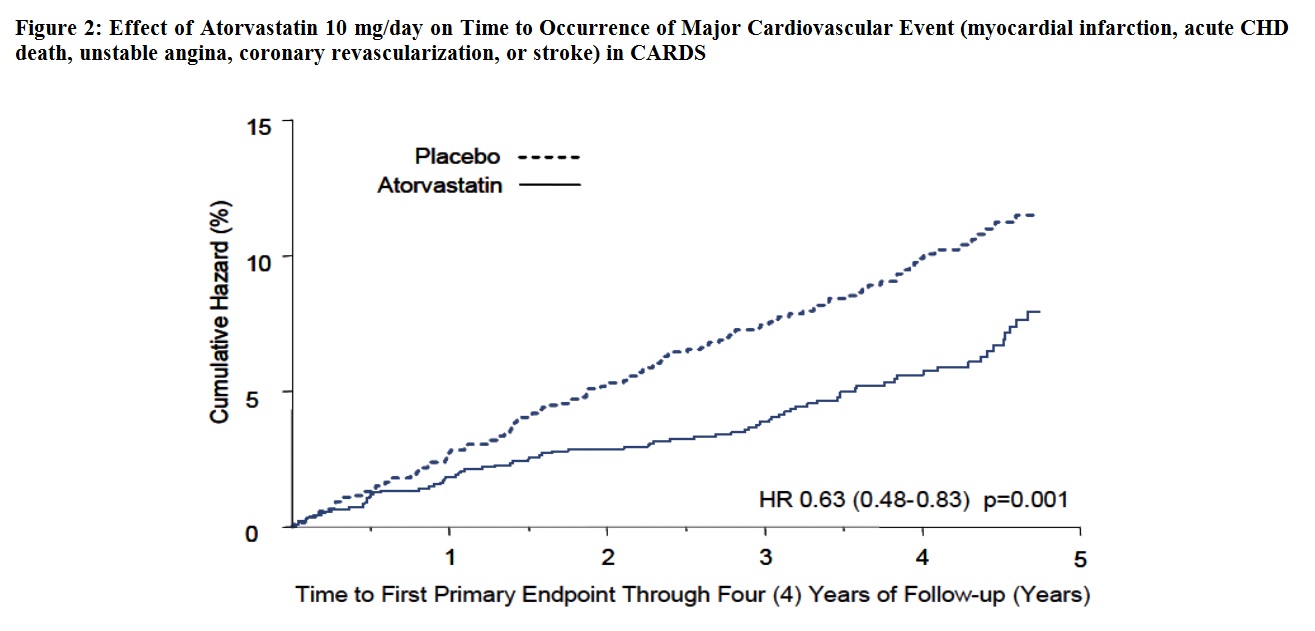
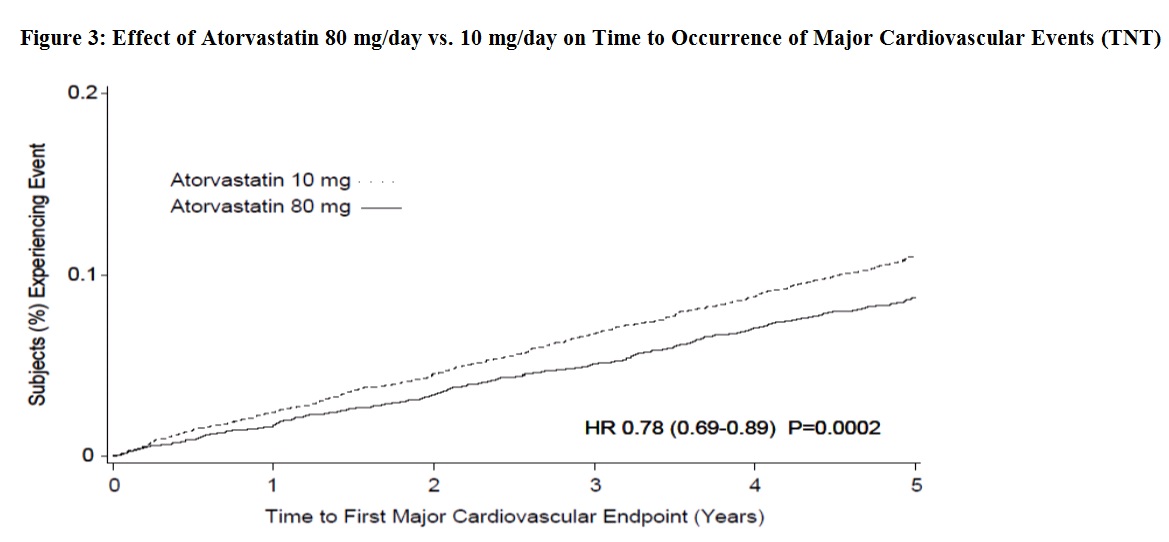
14 CLINICAL STUDIESPrevention of Cardiovascular Disease - In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), the effect of atorvastatin on fatal and non-fatal coronary heart disease was assessed in 10,305 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAtorvastatin calcium tablets are supplied as follows: 10 mg atorvastatin: white to off-white, oval shaped, biconvex, film coated tablets debossed with "LA37" on one side and plain on the other ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Myopathy and Rhabdomyolysis - Advise patients that atorvastatin may cause myopathy and rhabdomyolysis. Inform ...
-
PATIENT INFORMATIONPatient Information - Atorvastatin Calcium - (a tor'' va stat' in kal' see um) Tablets, USP, for oral use - What are atorvastatin calcium tablets? Atorvastatin calcium tablets ...
-
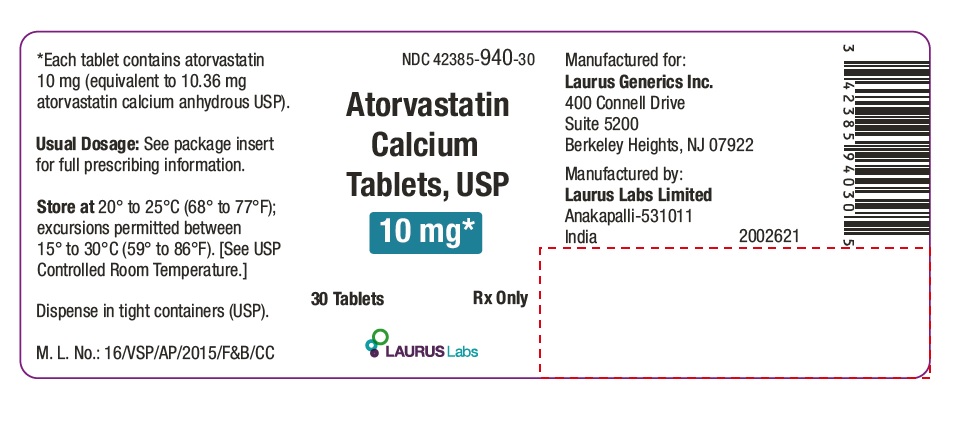
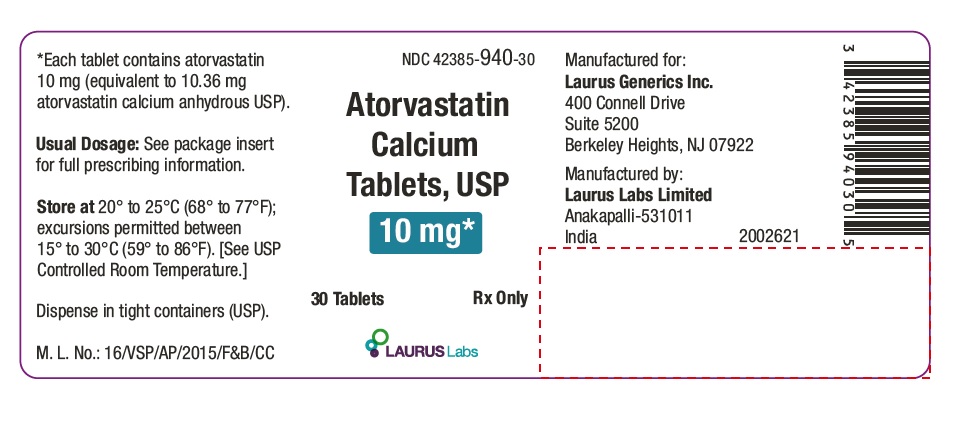
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mg - Container Label (30's count)NDC 42385-940-30 - Atorvastatin Calcium Tablets, USP 10 mg* 30 Tablets Rx Only - Laurus Labs
-
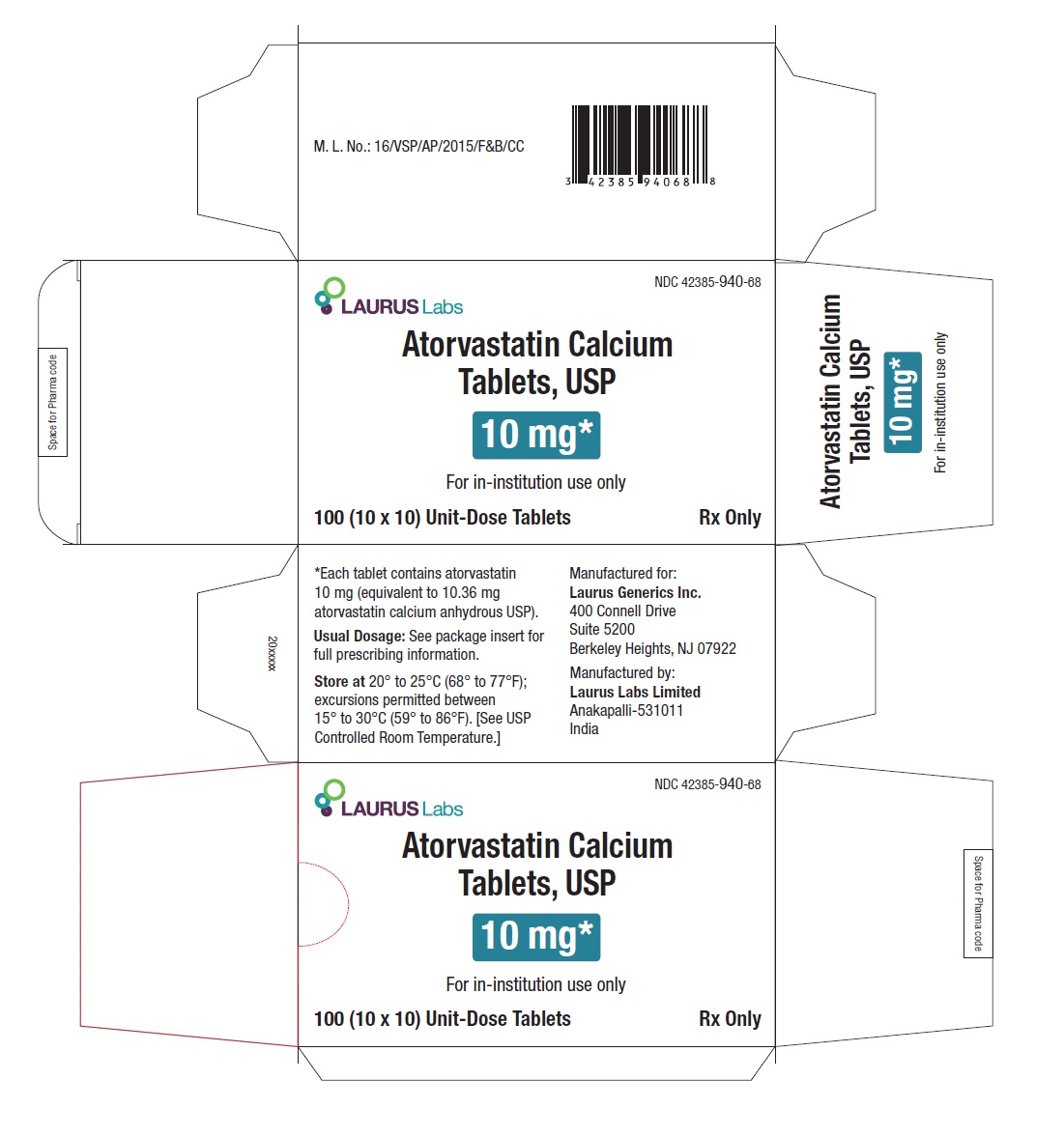
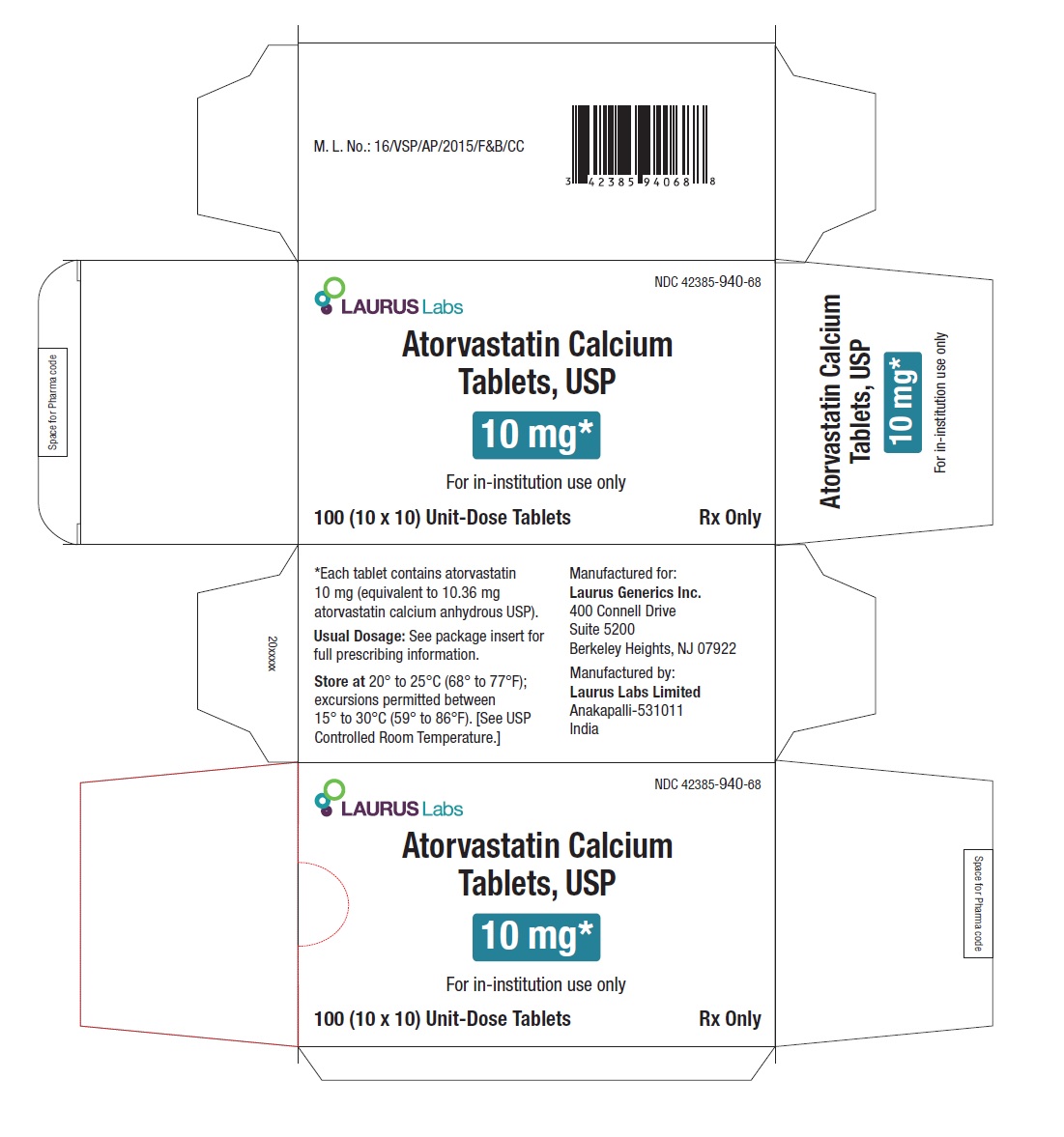
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -10 mg - Blister Carton - 100 (10 x 10) Unit-Dose TabletsNDC 42385-940-68 - Laurus Labs - Atorvastatin Calcium Tablets, USP 10 mg* For in-institution use only - 100 (10 x 10) Unit-Dose Tablets Rx Only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mg - Blister (1 x 10's count)Rx Only NDC 42385-940-10 - Atorvastatin Calcium Tablet, USP 10 mg - Mfd.by: Laurus Labs Limited - M.L.No.:16/VSP/AP/2015/F&B/CC
-
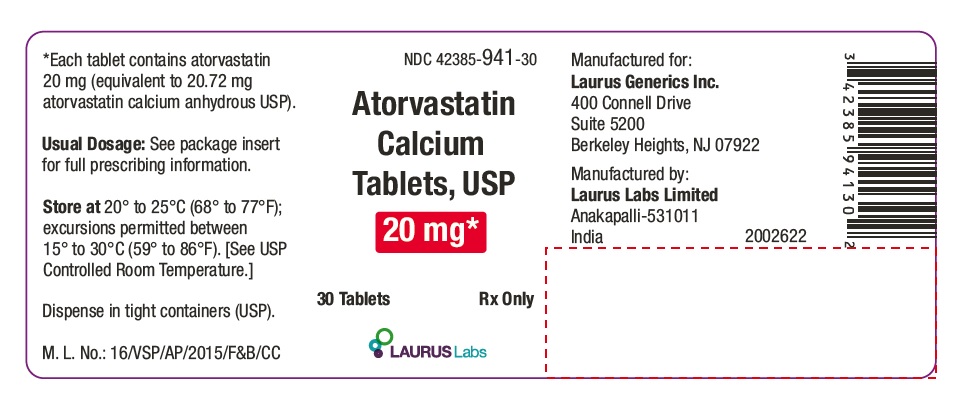
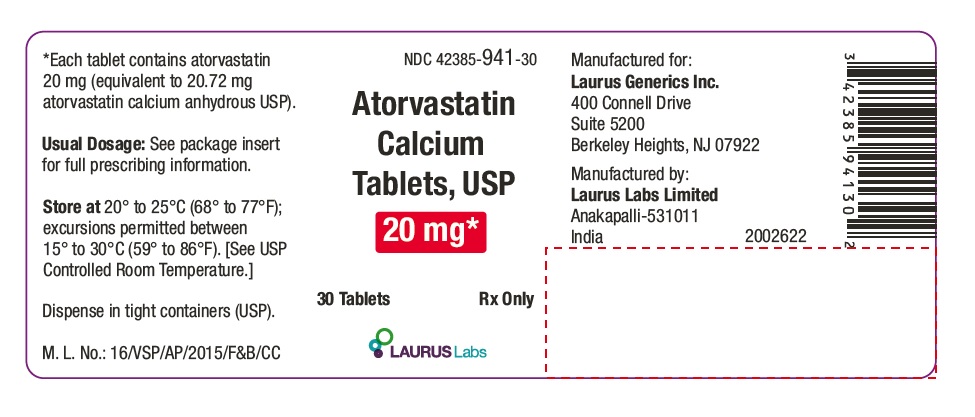
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Container Label (30's count)NDC 42385-941-30 - Atorvastatin Calcium Tablets, USP 20 mg* 30 Tablets Rx Only - Laurus Labs
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Blister Carton - 100 (10 x 10) Unit-Dose TabletsNDC 42385-941-68 - Laurus Labs - Atorvastatin Calcium Tablets, USP 20 mg* For in-institution use only - 100 (10 x 10) Unit-Dose Tablets Rx Only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg - Blister (1 x 10's count)Rx Only NDC 42385-941-10 - Atorvastatin Calcium Tablet, USP 20 mg - Mfd.by: Laurus Labs Limited - M.L.No.:16/VSP/AP/2015/F&B/CC
-
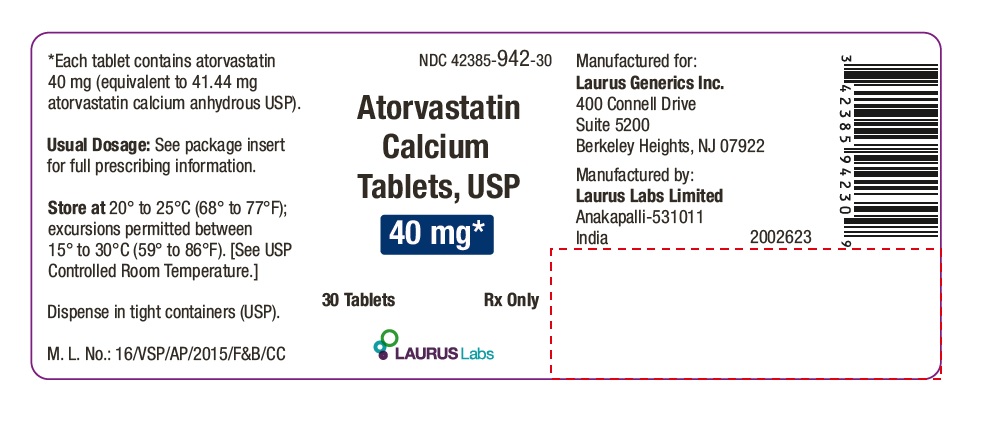
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Container Label (30's count)NDC 42385-942-30 - Atorvastatin Calcium Tablets, USP 40 mg* 30 Tablets Rx Only - Laurus Labs
-
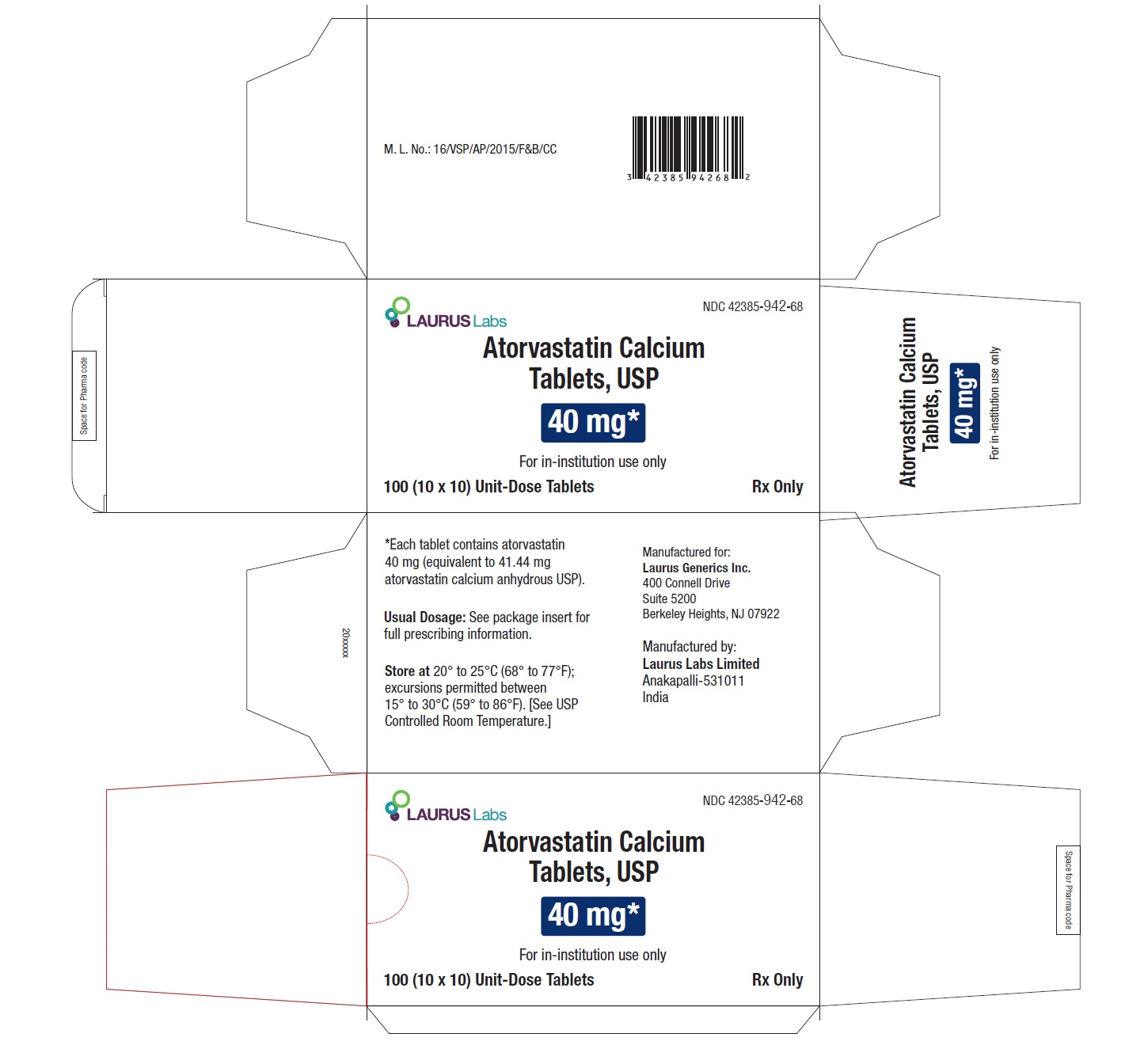
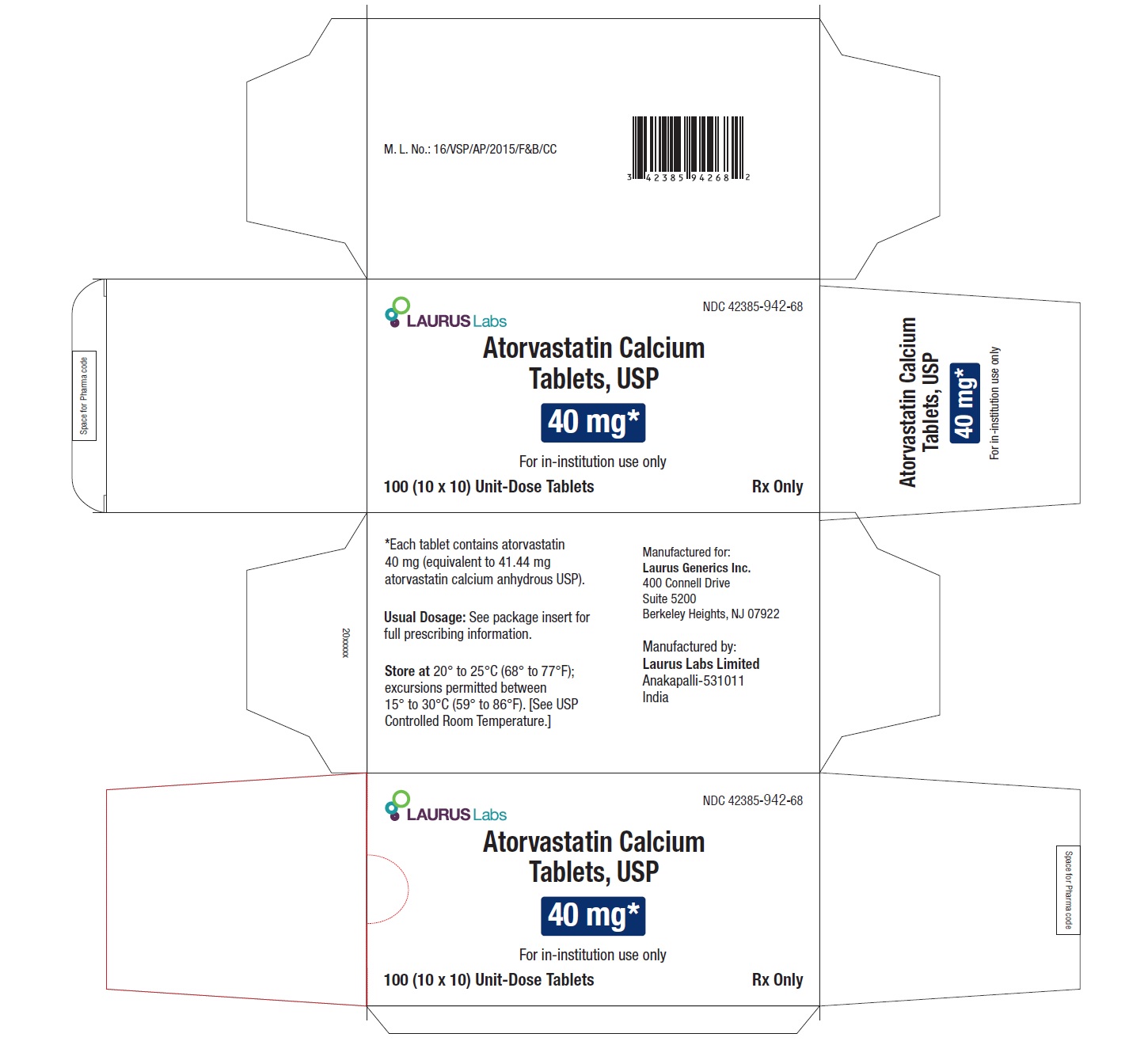
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Blister Carton - 100 (10 x 10) Unit-Dose TabletsNDC 42385-942-68 - Laurus Labs - Atorvastatin Calcium Tablets, USP 40 mg* For in-institution use only - 100 (10 x 10) Unit-Dose Tablets Rx Only
-
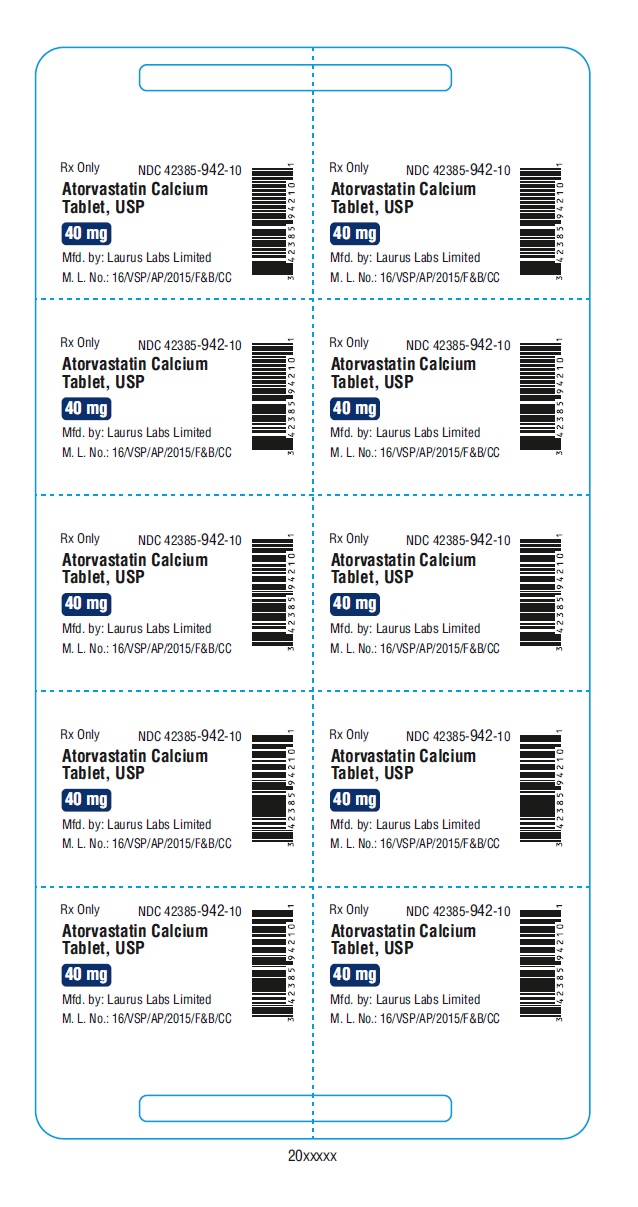
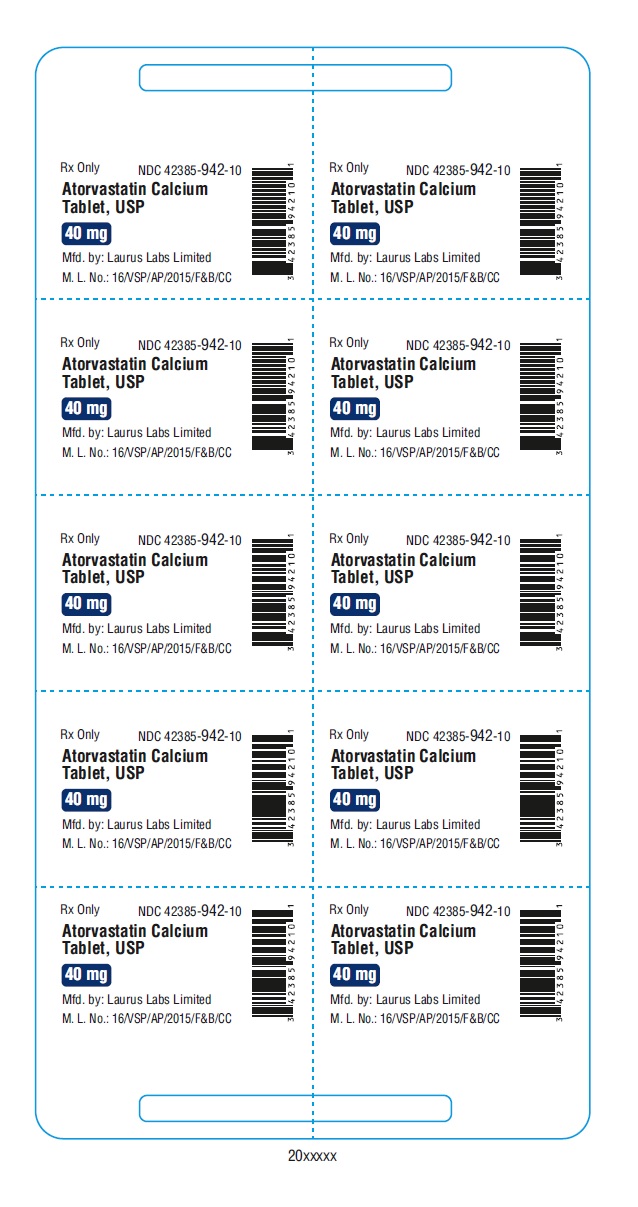
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 40 mg - Blister (1 x 10's count)Rx Only NDC 42385-942-10 - Atorvastatin Calcium Tablet, USP 40 mg - Mfd.by: Laurus Labs Limited - M.L.No.:16/VSP/AP/2015/F&B/CC
-
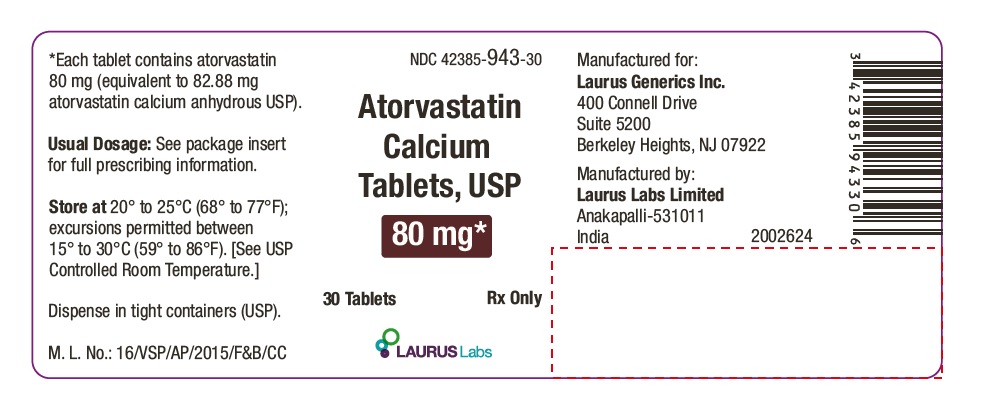
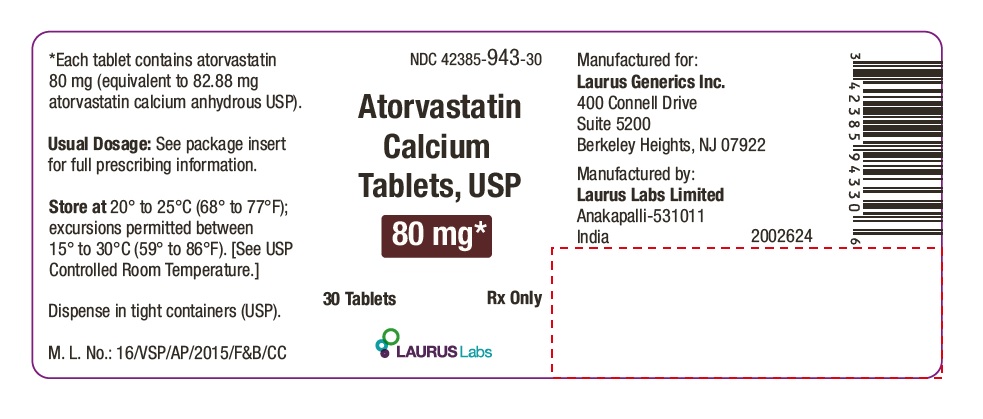
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Container Label (30's count)NDC 42385-943-30 - Atorvastatin Calcium Tablets, USP 80 mg* 30 Tablets Rx Only - Laurus Labs
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister Carton - 64 (8 x 8) Unit-Dose TabletsNDC 42385-943-64 - Laurus Labs - Atorvastatin Calcium Tablets, USP 80 mg* For in-institution use only - 64 (8 x 8) Unit-Dose Tablets Rx Only
-
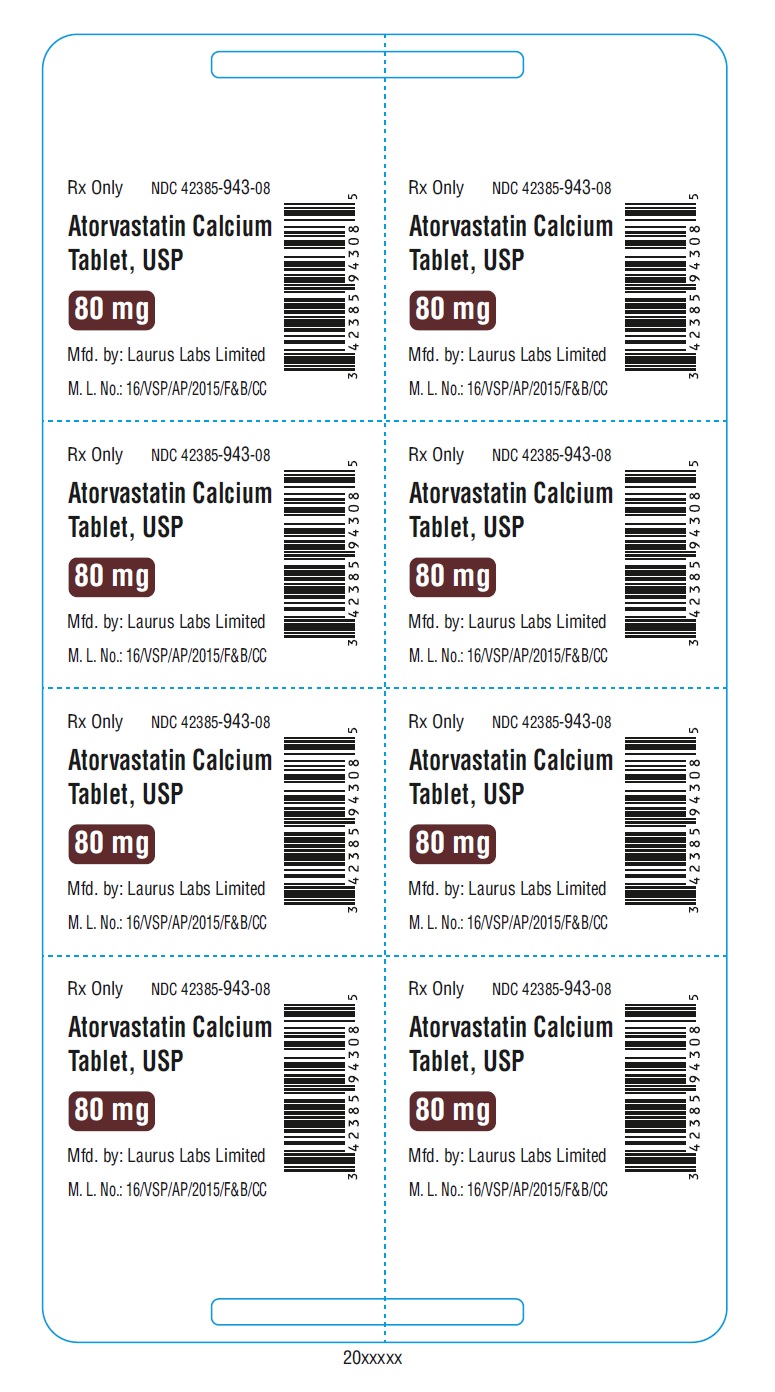
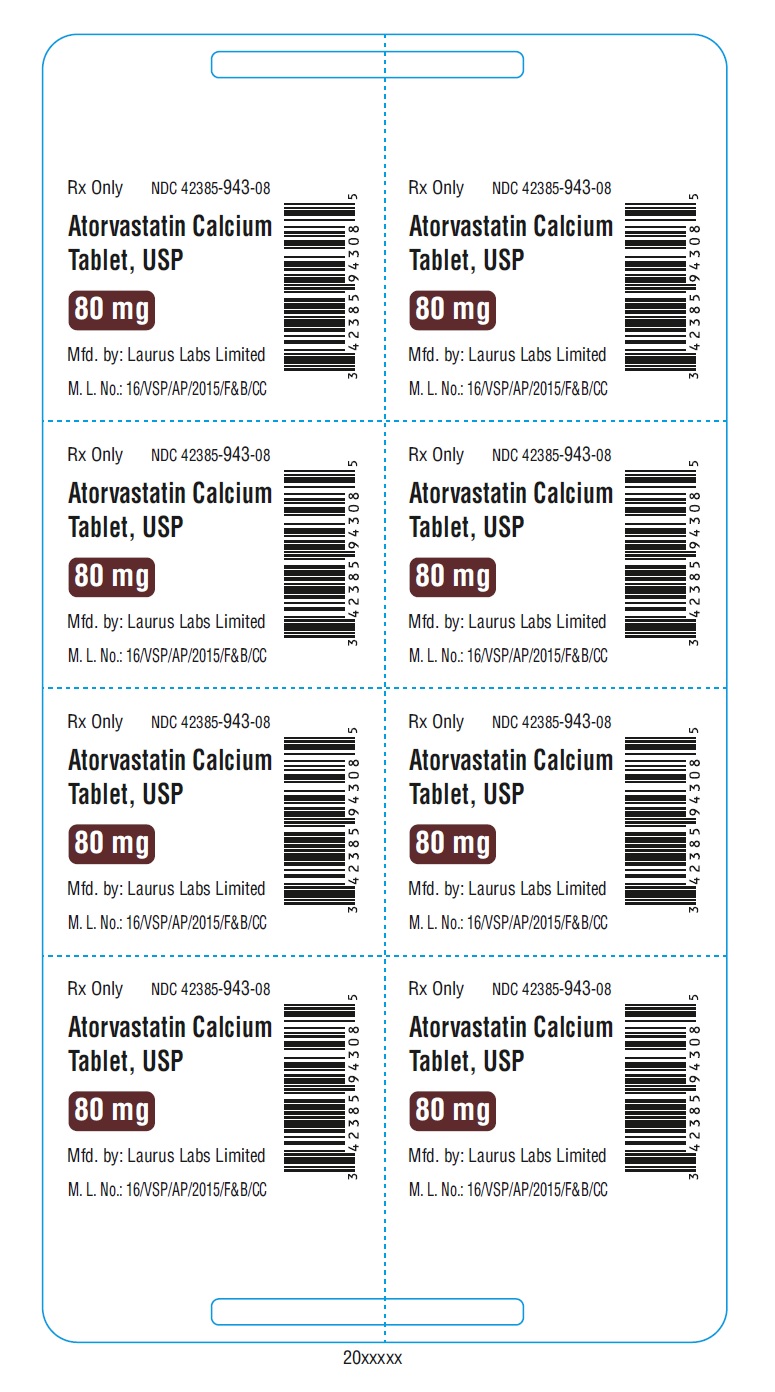
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister (1 x 8's count)Rx Only NDC 42385-943-08 - Atorvastatin Calcium Tablet, USP 80 mg - Mfd.by: Laurus Labs Limited - M.L.No.:16/VSP/AP/2015/F&B/CC
-
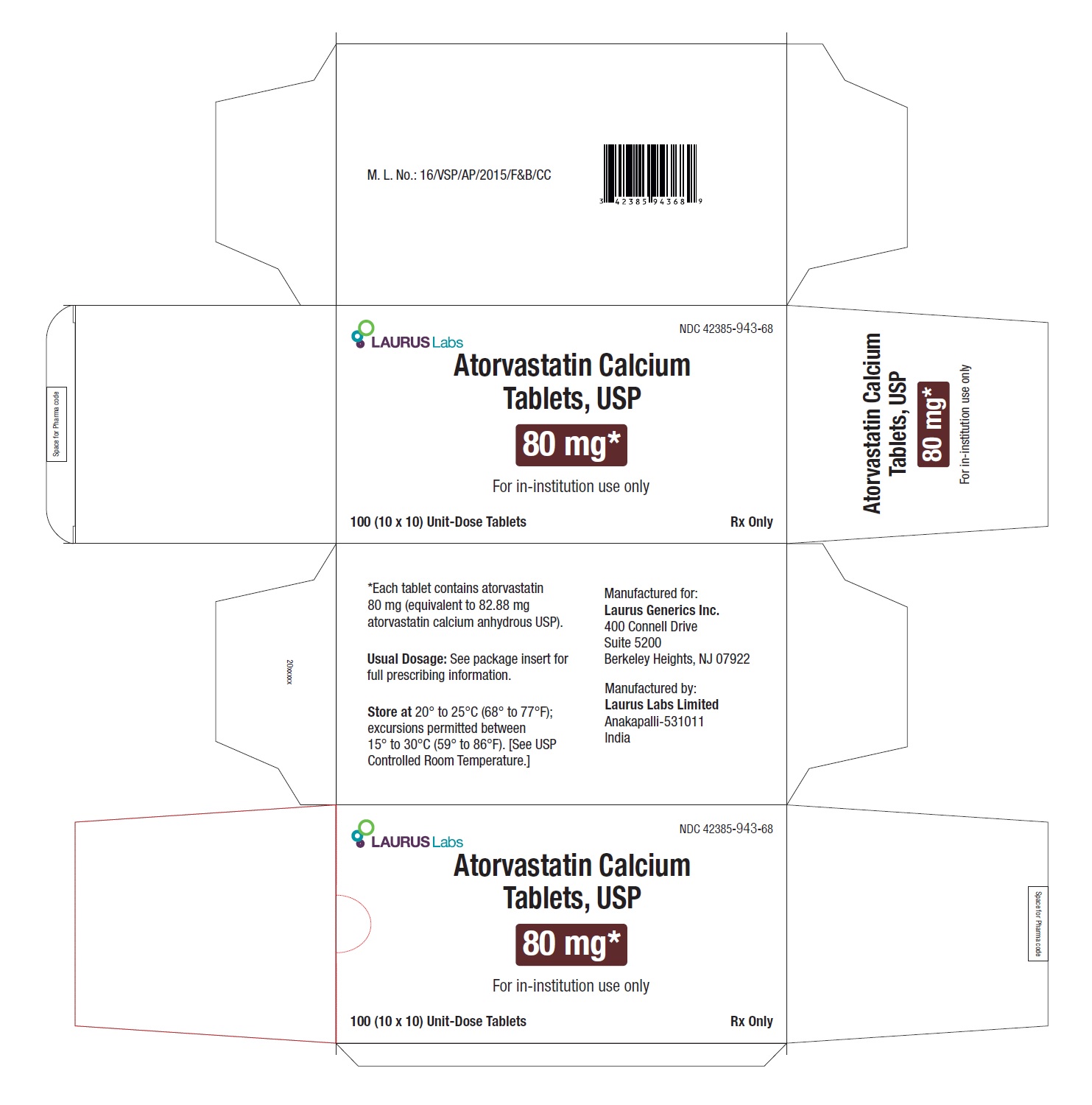
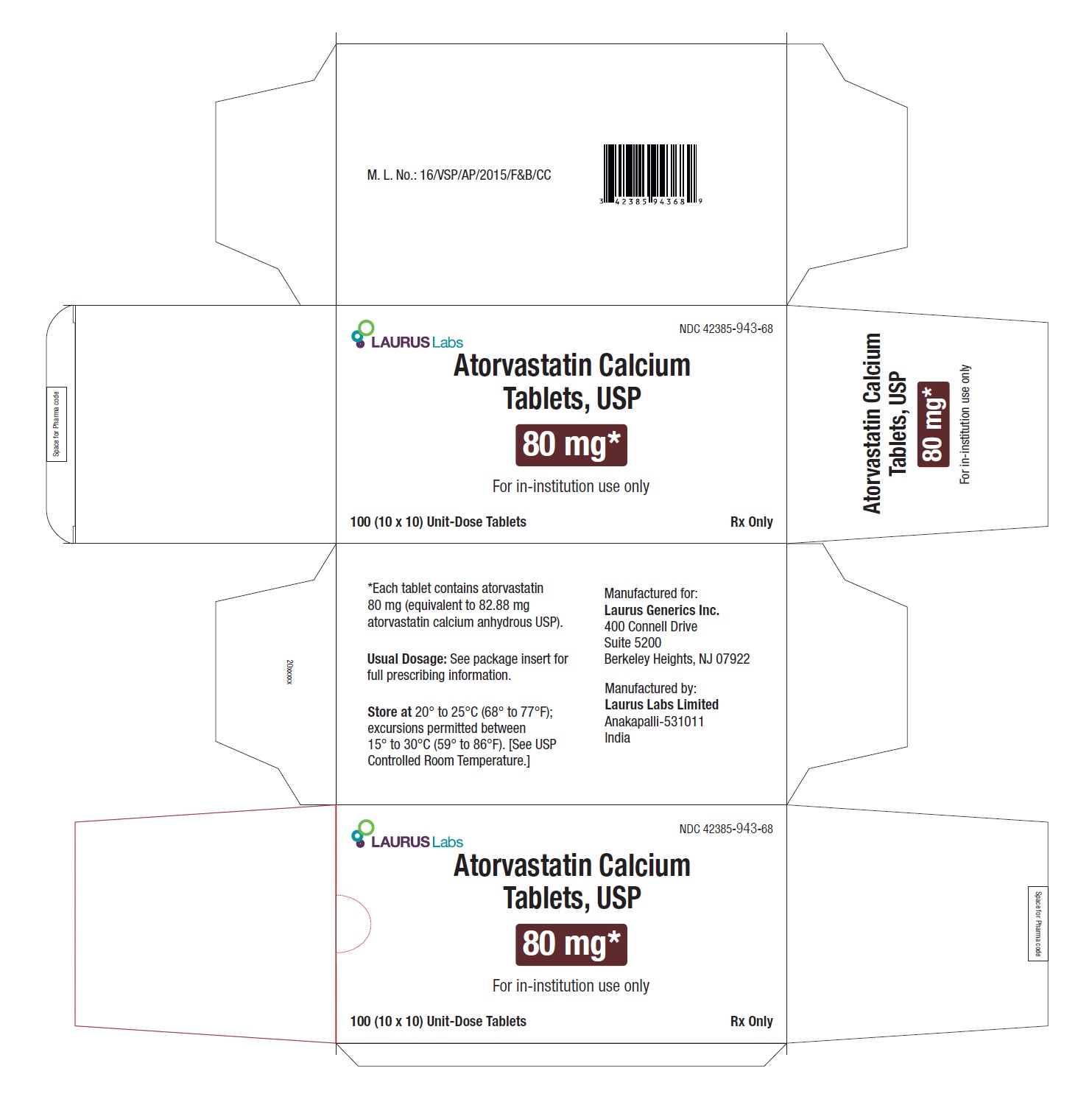
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister Carton - 100 (10 x 10) Unit-Dose TabletsNDC 42385-943-68 - Laurus Labs - Atorvastatin Calcium Tablets, USP 80 mg* For in-institution use only - 100 (10 x 10) Unit-Dose Tablets Rx Only
-
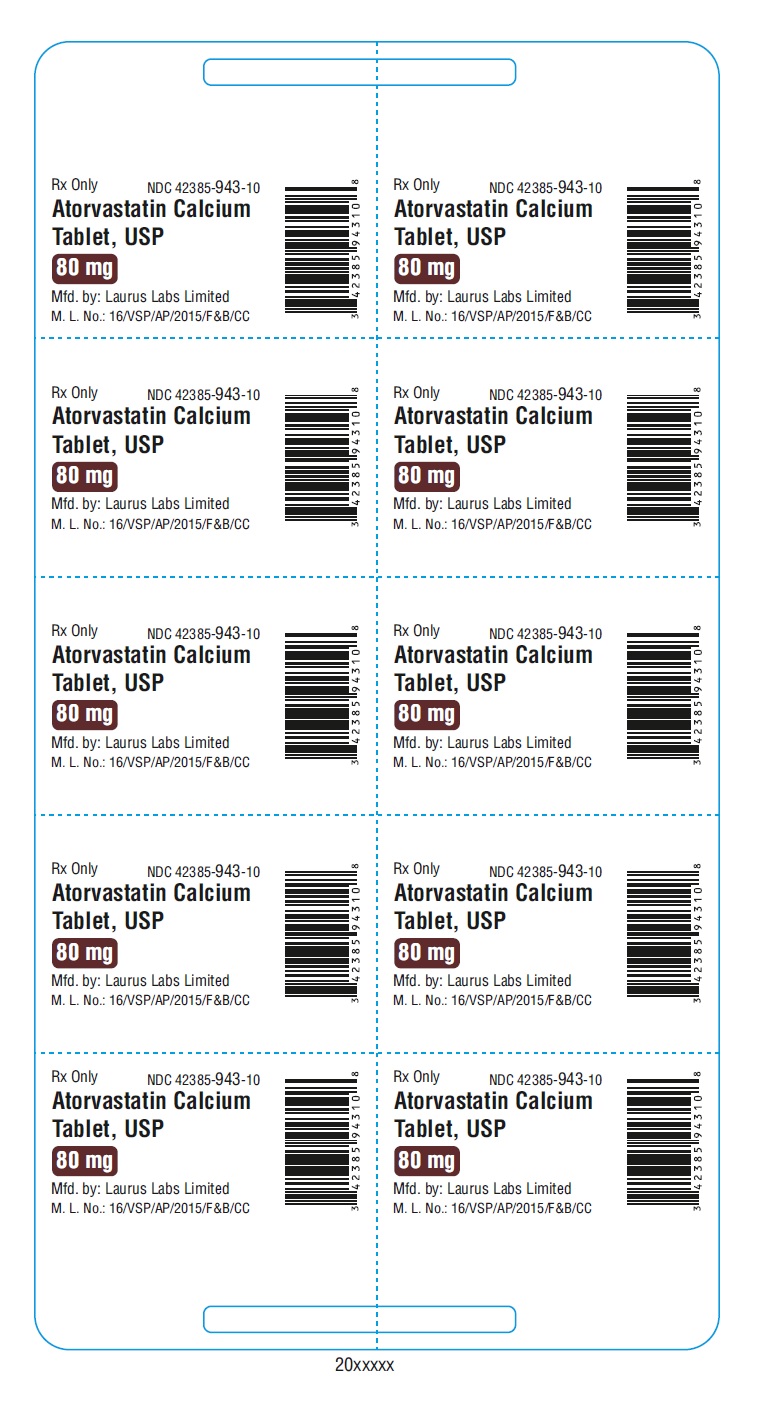
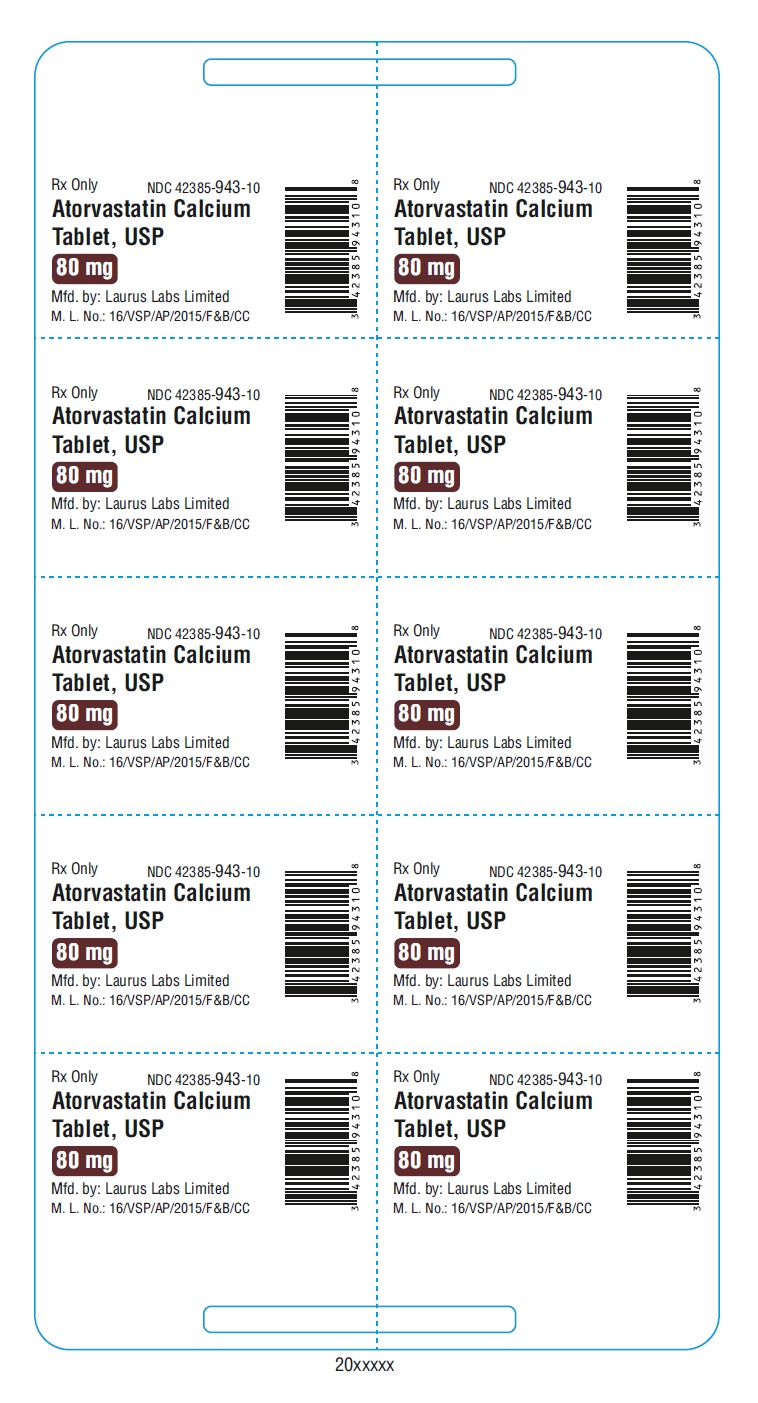
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 80 mg - Blister (1 x 10's count)Rx Only NDC 42385-943-10 - Atorvastatin Calcium Tablet, USP 80 mg - Mfd.by: Laurus Labs Limited - M.L.No.:16/VSP/AP/2015/F&B/CC
-
INGREDIENTS AND APPEARANCEProduct Information