Label: SODIUM SULFACETAMIDE, SULFUR- sodium sulfacetamide and sulfur cream
- NDC Code(s): 42192-139-01, 42192-149-02
- Packager: Acella Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
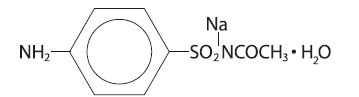
DESCRIPTIONDESCRIPTION: Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: Sodium Sulfacetamide exhibits antibacterial activity. It is believed to block bacterial growth by acting as a competitive antagonist of para-aminobenzoic acid (PABA). While ...
-
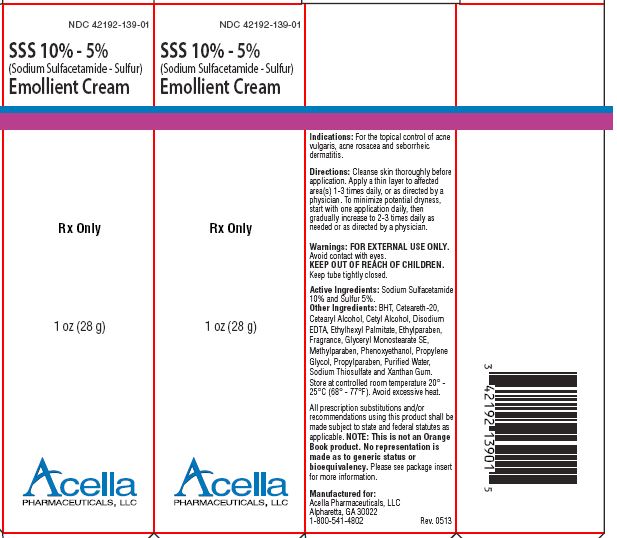
INDICATIONS & USAGEINDICATIONS: Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream is indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
-
CONTRAINDICATIONSCONTRAINDICATIONS: Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this ...
-
WARNINGSWARNINGS: Although rare, sensitivity to sodium sulfacetaminde may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to ...
-
GENERAL PRECAUTIONSPRECAUTIONS: General - If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or ...
-
INFORMATION FOR PATIENTSInformation for patients - Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and ...
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITYCarcinogenesis, Mutagenesis and Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential.
-
PREGNANCYPREGNANCY: Category C. Animal reproduction studies have not been conducted with Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream. It is not known whether Sodium Sulfacetamide 10% Sulfur 5 ...
-
NURSING MOTHERSNURSING MOTHERS: It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream. However, small amounts of ...
-
PEDIATRIC USEPEDIATRIC USE: Safety and effectiveness in children under the age of 12 have not been established.
-
ADVERSE REACTIONSADVERSE REACTIONS: Although rare, sodium sulfacetamide may cause local irritation.
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Cleanse skin thoroughly before application. Apply a thin layer to affected areas 1-3 times daily or as directed by a physician. To minimize potential dryness, start with ...
-
HOW SUPPLIEDHOW SUPPLIED: Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream is available in 2 oz (57 g) tubes, NDC 42192-149-02 and SSS 10% - 5% (Sodium Sulfacetamide - Sulfur) Emollient Cream in 1 oz (28 g ...
-
STORAGE AND HANDLINGStore at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [see USP Controlled Room Temperature]
-
SPL UNCLASSIFIED SECTIONKEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. All prescription substitutions using this product shall be made subject to state and federal statutes as applicable. NOTE ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 28 g carton labelNDC 42192-139-01 - SSS 10% - 5% (Sodium Sulfacetamide - Sulfur) Emollient Cream - Rx Only - 1 oz (28 g) Acella - PHARMACEUTICALS, LLC
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 57 g carton label42192-149-02 - Sodium Sulfacetamide 10% Sulfur 5% Emollient Cream - Net wt. 2 oz (57 g) Acella - PHARMACEUTICALS, LLC
-
INGREDIENTS AND APPEARANCEProduct Information