Label: LEVOFLOXACIN tablet, film coated
-
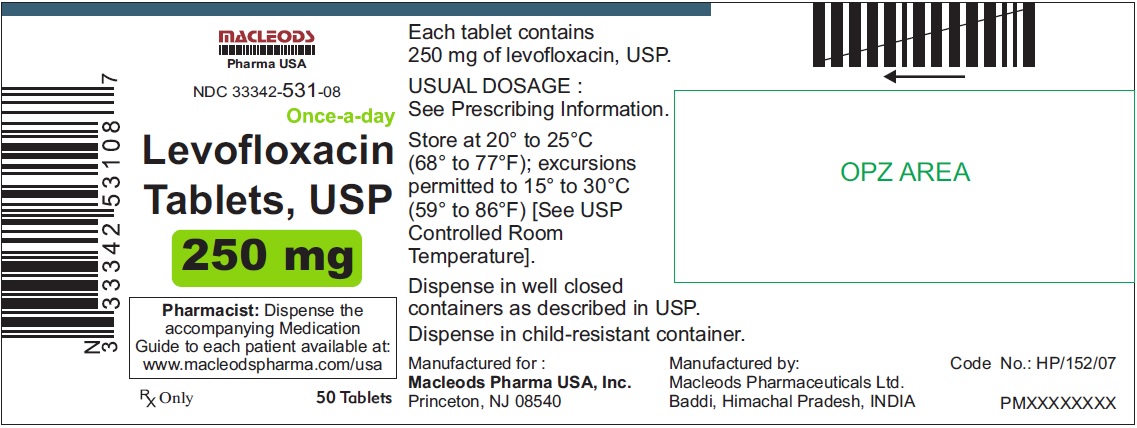
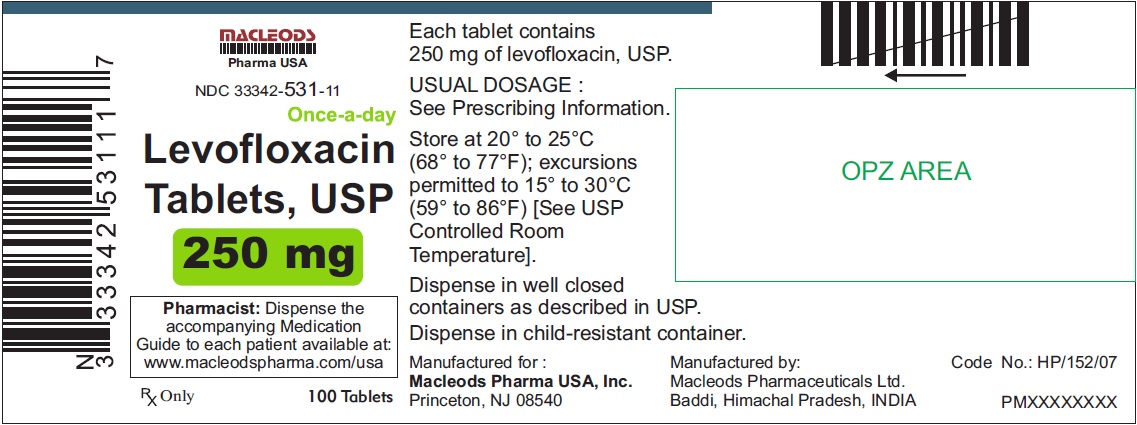
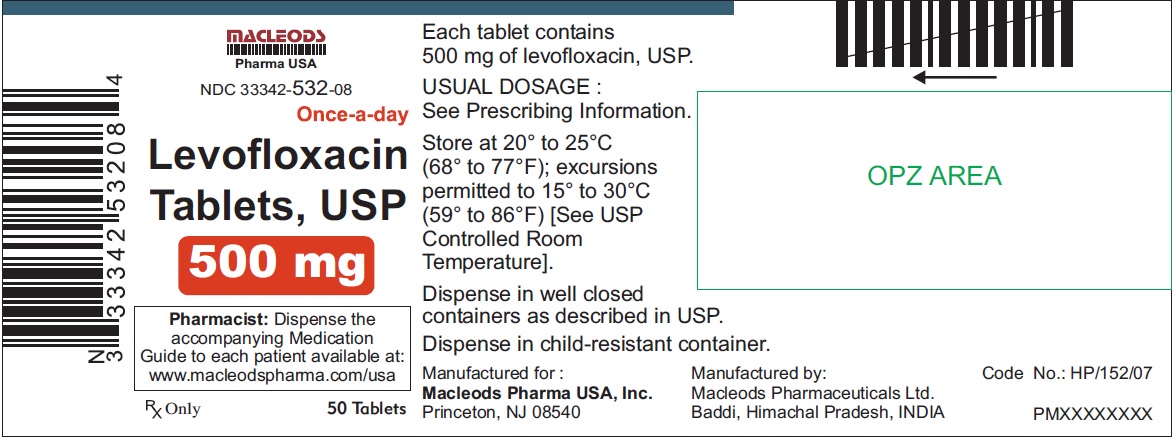
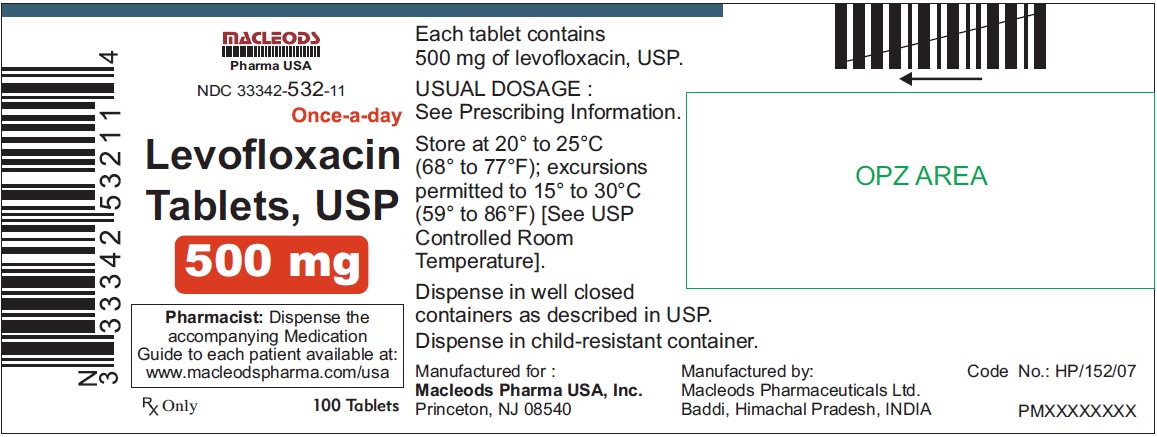
NDC Code(s):
33342-531-08,
33342-531-11,
33342-531-12,
33342-531-31, view more33342-532-08, 33342-532-11, 33342-532-12, 33342-532-31, 33342-533-11, 33342-533-12, 33342-533-31, 33342-533-32
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOFLOXACIN TABLETS, safely and effectively. See full prescribing information for LEVOFLOXACIN TABLETS. LEVOFLOXACIN tablets, for ...These highlights do not include all the information needed to use LEVOFLOXACIN TABLETS, safely and effectively. See full prescribing information for LEVOFLOXACIN TABLETS.
LEVOFLOXACIN tablets, for oral use
Initial U.S. Approval: 1996WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
See full prescribing information for complete boxed warning.
Fluoroquinolones, including levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together (5.1), including:
o Tendinitis and tendon rupture (5.2)
o Peripheral neuropathy (5.3)
o Central nervous system effects (5.4)
Discontinue levofloxacin immediately and avoid the use of fluoroquinolones, including levofloxacin, in patients who experience any of these serious adverse reactions (5.1)
Fluoroquinolones, including levofloxacin, may exacerbate muscle weakness in patients with myasthenia gravis. Avoid levofloxacin in patients with a known history of myasthenia gravis [see Warnings and Precautions (5.5)].
Because fluoroquinolones, including levofloxacin, have been associated with serious adverse reactions (5.1-5.15), reserve levofloxacin for use in patients who have no alternative treatment options for the following indications:
o Uncomplicated urinary tract infection (1.12)
o Acute bacterial exacerbation of chronic bronchitis (1.13)
o Acute bacterial sinusitis (1.14)
RECENT MAJOR CHANGES
• Indications and Usage – Oral solution and Injection Dosage Forms Removed (1) 7/2018
• Dosage and Administration – Oral Solution and Injection Dosage Forms Removed (2) 7/2018
• Warnings and Precautions,-Central Nervous System Effects (5.4) 10/2018
• Warnings and Precautions,- Risk of Aortic Aneurysm and Dissection (5.9) 05/2019
• Warnings and Precautions,- Blood Glucose Disturbances (5.13) 10/2018
INDICATIONS AND USAGE
Levofloxacin tablets are fluoroquinolone antibacterial indicated in adults (18 years of age and older) with infections caused by designated, susceptible bacteria and in pediatric patients where indicated (1, 12.4).
• Pneumonia: Nosocomial (1.1) and Community Acquired (1.2, 1.3)
• Skin and Skin Structure Infections (SSSI): Complicated (1.4) and Uncomplicated (1.5)
• Chronic bacterial prostatitis (1.6)
• Inhalational Anthrax, Post-Exposure in adult and pediatric patients (1.7)
• Plague in adult and pediatric patients (1.8)
• Urinary Tract Infections (UTI): Complicated (1.9, 1.10) and Uncomplicated (1.12)
• Acute Pyelonephritis (1.11)
• Acute Bacterial Exacerbation of Chronic Bronchitis (1.13)
• Acute Bacterial Sinusitis (1.14)Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of levofloxacin tablets and other antibacterial drugs, levofloxacin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria (1.15).
DOSAGE AND ADMINISTRATION
• Administer levofloxacin tablets to pediatric patients weighing 30 kg and greater only (2.1, 2.2).
• Levofloxacin Tablets cannot be administered to pediatric patients who weigh less than 30 kg because of the limitations of the available strengths. Alternative formulations of levofloxacin may be considered for pediatric patients who weigh less than 30 kg (2.2).Dosage in Adult and Pediatric Patients with Creatinine Clearance greater than or equal to 50 mL/minute (2.1. 2.2)
Type of Infection
Dose Every 24 hours
Duration (days)
Nosocomial Pneumonia (1.1)
750 mg
7 to 14
Community Acquired Pneumonia (1.2)
500 mg
7 to 14
Community Acquired Pneumonia (1.3)
750 mg
5
Complicated SSSI (1.4)
750 mg
7 to 14
Uncomplicated SSSI (1.5)
500 mg
7 to 10
Chronic Bacterial Prostatitis (1.6)
500 mg
28
Inhalational Anthrax (Post-Exposure) (1.7) Adults and Pediatric Patients 50 kg or greater Pediatric Patients 30 kg to less than 50 kg (2.2)
500 mg
250 mg every 12 hours
60
60
Plague (1.8) Adults and Pediatric Patients 50 kg or greater Pediatric Patients 30 kg to less than 50 kg (2.2)
500 mg
250 mg every 12 hours
10 to 14
10 to 14
Complicated UTI (1.9) or Acute Pyelonephritis (1.11)
750 mg
5
Complicated UTI (1.10) or Acute Pyelonephritis (1.11)
250 mg
10
Uncomplicated UTI (1.12)
250 mg
3
Acute Bacterial Exacerbation of Chronic Bronchitis (1.13)
500 mg
7
Acute Bacterial Sinusitis (1.14)
750 mg
5
500 mg
10 to 14
• Adjust dose for creatinine clearance less than 50 mL/minute (2.3, 8.6, 12.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 250 mg, 500 mg, and 750 mg (3)
WARNINGS AND PRECAUTIONS
- Anaphylactic reactions and allergic skin reactions, serious, occasionally fatal, may occur after first dose (4, 5.7)
- Hematologic (including agranulocytosis, thrombocytopenia), and renal toxicities may occur after multiple doses (5.6)
- Hepatotoxicity: Severe, and sometimes fatal, hepatotoxicity has been reported. Discontinue immediately if signs and symptoms of hepatitis occur (5.8)
- Clostridium difficile-associated colitis: evaluate if diarrhea occurs (5.10)
- Prolongation of the QT interval and isolated cases of torsade de pointes have been reported. Avoid use in patients with known prolongation, those with hypokalemia, and with other drugs that prolong the QT interval (5.11, 8.5)
ADVERSE REACTIONS
The most common reactions (≥3%) were nausea, headache, diarrhea, insomnia, constipation and dizziness (6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA, Inc., at 1-888-943-3210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
Interacting Drug Interacting Drug Interaction Interaction Interacting Drug Interaction Multivalent cation-containing products including antacids, metal cations or didanosine
Absorption of levofloxacin is decreased when the tablets are taken within 2 hours of these products (2.4, 7.1)
Warfarin
Effect may be enhanced. Monitor prothrombin time, INR and watch for bleeding (7.2)
Antidiabetic agents
Carefully monitor blood glucose (5.13, 7.3)
USE IN SPECIFIC POPULATIONS
- Geriatrics: Severe hepatotoxicity has been reported. The majority of reports describe patients 65 years of age or older (5.8,8.5, 17). May have increased risk of tendinopathy (including rupture), especially with concomitant corticosteroid use (5.2, 8.5, 17). May be more susceptible to prolongation of the QT interval. (5.11, 8.5, 17).
- Pediatrics: Musculoskeletal disorders (arthralgia, arthritis, tendinopathy, and gait abnormality) seen in more levofloxacin treated patients than in comparator. Shown to cause arthropathy and osteochondrosis in juvenile animals (5.12, 8.4, 13.2). Safety in pediatric patients treated for more than 14 days has not been studied. Risk-benefit appropriate only for the treatment of inhalational anthrax (postexposure) (1.7, 2.2, 8.4, 14.9) and plague (1.8, 2.2, 8.4, 14.10)
- Lactation: Breastfeeding is not recommended during treatment, but a lactating woman may pump and discard breastmilk during treatment and an additional 2 days after the last dose. In patients treated for inhalational anthrax (post exposure), consider the risks and benefits of continuing breastfeeding.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
1 INDICATIONS & USAGE
1.1 Nosocomial Pneumonia
1.2 Community-Acquired Pneumonia: 7 to 14 day Treatment Regimen
1.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
1.4 Complicated Skin and Skin Structure Infections
1.5 Uncomplicated Skin and Skin Structure Infections
1.6 Chronic Bacterial Prostatitis
1.7 Inhalational Anthrax (Post-Exposure)
1.8 Plague
1.9 Complicated Urinary Tract Infections: 5-day Treatment Regimen
1.10 Complicated Urinary Tract Infections: 10-day Treatment Regimen
1.11 Acute Pyelonephritis: 5 or 10-day Treatment Regimen
1.12 Uncomplicated Urinary Tract Infections
1.13 Acute Bacterial Exacerbation of Chronic Bronchitis
1.14 Acute Bacterial Sinusitis: 5-day and 10-14day Treatment Regimens
1.15 Usage
2 DOSAGE & ADMINISTRATION
2.1 Dosage of Levofloxacin Tablets in Adult Patients with Creatinine Clearance > 50mL/minute
2.2 Dosage of Levofloxacin Tablets in Pediatric Patients with Inhalational Anthrax or Plague
2.3 Dosage Adjustment in Adults with Renal Impairment
2.4 Drug Interaction With Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
2.5 Important Administration Instructions
2.6 Hydration for Patients Receiving Levofloxacin Tablets
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects
5.2 Tendinitis and Tendon Rupture
5.3 Peripheral Neuropathy
5.4 Central Nervous System Effects
5.5 Exacerbation of Myasthenia Gravis
5.6 Other Serious and Sometimes Fatal Adverse Reactions
5.7 Hypersensitivity Reactions
5.8 Hepatotoxicity
5.9 Risk of Aortic Aneurysm and Dissection
5.10 Clostridium difficile- Associated Diarrhea
5.11 Prolongation of the QT Interval
5.12 Musculoskeletal Disorders in Pediatric Patients and Arthropathic Effects in Animals
5.13 Blood Glucose Disturbances
5.14 Photosensitivity/ Phototoxicity
5.15 Development of Drug Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
7.2 Warfarin
7.3 Antidiabetic Agents
7.4 Non-Steroidal Anti-Inflammatory Drugs
7.5 Theophylline
7.6 Cyclosporine
7.7 Digoxin
7.8 Probenecid and Cimetidine
7.9 Interactions with Laboratory or Diagnostic Testing
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
13.2 Animal Toxicology & or Pharmacology
14 CLINICAL STUDIES
14.1 Nosocomial Pneumonia
14.2 Community-Acquired Pneumonia: 7-14 day Treatment Regimen
14.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
14.4 Acute Bacterial Sinusitis: 5-day and 10-14 day Treatment Regimens
14.5 Complicated Skin and Skin Structure Infections
14.6 Chronic Bacterial Prostatitis
14.7 Complicated Urinary Tract Infections and Acute Pyelonephritis: 5-day Treatment Regimen
14.8 Complicated Urinary Tract Infections and Acute Pyelonephritis: 10-day Treatment Regimen
14.9 Inhalational Anthrax (Post-Exposure)
14.10 Plague
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
• Fluoroquinolones, including levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together [see Warnings and Precautions (5.1)], including:
o Tendinitis and tendon rupture [see Warnings and Precautions (5.2)]
o Peripheral neuropathy [see Warnings and Precautions (5.3)]
o Central nervous system effects [see Warnings and Precautions (5.4)]Discontinue levofloxacin immediately and avoid the use of fluoroquinolones, including levofloxacin, in patients who experience any of these serious adverse reactions [see Warnings and Precautions (5.1)]
• Fluoroquinolones, including levofloxacin, may exacerbate muscle weakness in patients with myasthenia gravis. Avoid levofloxacin in patients with a known history of myasthenia gravis [see Warnings and Precautions (5.5)].
• Because fluoroquinolones, including levofloxacin, have been associated with serious adverse reactions [see Warnings and Precautions (5.1-5.15)], reserve levofloxacin for use in patients who have no alternative treatment options for the following indications:
o Uncomplicated urinary tract infection [see Indications and Usage (1.12)]
o Acute bacterial exacerbation of chronic bronchitis [see Indications and Usage (1.13)]
o Acute bacterial sinusitis [see Indications and Usage (1.14)].
Close -
1 INDICATIONS & USAGE1.1 Nosocomial Pneumonia - Levofloxacin tablets are indicated in adult patients for the treatment of nosocomial pneumonia due to methicillin-susceptible Staphylococcus aureus, Pseudomonas ...
1.1 Nosocomial Pneumonia
Levofloxacin tablets are indicated in adult patients for the treatment of nosocomial pneumonia due to methicillin-susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended [see Clinical Studies (14.1)].
1.2 Community-Acquired Pneumonia: 7 to 14 day Treatment Regimen
Levofloxacin tablets are indicated in adult patients for the treatment of community-acquired pneumonia due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multi-drug-resistant Streptococcus pneumoniae [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae [see Dosage and Administration (2.1) and Clinical Studies (14.2)].
MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
1.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
Levofloxacin tablets are indicated in adult patients for the treatment of community-acquired pneumonia due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, or Chlamydophila pneumoniae [see Dosage and Administration (2.1)and Clinical Studies (14.3)].
1.4 Complicated Skin and Skin Structure Infections
Levofloxacin tablets are indicated in adult patients for the treatment of complicated skin and skin structure infections due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, or Proteus mirabilis [see Clinical Studies (14.5)].
1.5 Uncomplicated Skin and Skin Structure Infections
Levofloxacin tablets are indicated in adult patients for the treatment of uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections, due to methicillin-susceptible Staphylococcus aureus, or Streptococcus pyogenes.
1.6 Chronic Bacterial Prostatitis
Levofloxacin tablets are indicated in adult patients for the treatment of chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis, or methicillin-susceptible Staphylococcus epidermidis [see Clinical Studies (14.6)].
1.7 Inhalational Anthrax (Post-Exposure)
Levofloxacin tablets are indicated for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis in adults and pediatric patients, 6 months of age and older [see Dosage and Administration (2.2)]. . The effectiveness of levofloxacin tablets are based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. Levofloxacin tablets has not been tested in humans for the post-exposure prevention of inhalation anthrax. The safety of levofloxacin tablets in adults for durations of therapy beyond 28 days or in pediatric patients for durations of therapy beyond 14 days has not been studied.
Prolonged levofloxacin tablets therapy should only be used when the benefit outweighs the risk [see Clinical Studies (14.9)].
1.8 Plague
Levofloxacin tablets are indicated for treatment of plague, including pneumonic and septicemic plague, due to Yersinia pestis (Y. pestis) and prophylaxis for plague in adults and pediatric patients, 6 months of age and older [see Dosage and Administration (2.2)]. Efficacy studies of levofloxacin tablets could not be conducted in humans with plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals [see Clinical Studies (14.10)].
1.9 Complicated Urinary Tract Infections: 5-day Treatment Regimen
Levofloxacin tablets are indicated in adult patients for the treatment of complicated urinary tract infections due to Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis [see Clinical Studies (14.7)].
1.10 Complicated Urinary Tract Infections: 10-day Treatment Regimen
Levofloxacin tablets are indicated in adult patients for the treatment of complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa [see Clinical Studies (14.8)].
1.11 Acute Pyelonephritis: 5 or 10-day Treatment Regimen
Levofloxacin tablets are indicated in adult patients for the treatment of acute pyelonephritis caused by Escherichia coli, including cases with concurrent bacteremia [see Clinical Studies (14.7, 14.8)].
1.12 Uncomplicated Urinary Tract Infections
Levofloxacin tablets are indicated in adult patients for the treatment of uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.
Because fluoroquinolones, including levofloxacin, have been associated with serious adverse reactions [see Warnings and Precautions (5.1-5.15)] and for some patients uncomplicated urinary tract infection is self-limiting, reserve levofloxacin tablets for treatment of uncomplicated urinary tract infections in patients who have no alternative treatment options.
1.13 Acute Bacterial Exacerbation of Chronic Bronchitis
Levofloxacin tablets are indicated in adult patients for the treatment of acute bacterial exacerbation of chronic bronchitis (ABECB) due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Because fluoroquinolones, including levofloxacin, have been associated with serious adverse reactions [see Warnings and Precautions (5.1-5.15)] and for some patients ABECB is self-limiting, reserve levofloxacin tablets for treatment of ABECB in patients who have no alternative treatment options.1.14 Acute Bacterial Sinusitis: 5-day and 10-14day Treatment Regimens
Levofloxacin tablets are indicated in adult patients for the treatment of acute bacterial sinusitis (ABS) due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis [see Clinical Studies (14.4)].
Because fluoroquinolones, including levofloxacin have been associated with serious adverse reactions [see Warnings and Precautions (5.1-5.15)] and for some patients ABS is self-limiting, reserve levofloxacin tablets for treatment of ABS in patients who have no alternative treatment options.
Close1.15 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of levofloxacin and other antibacterial drugs, levofloxacin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Culture and susceptibility testing
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin [see Microbiology (12.4)]. Therapy with levofloxacin tablets may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected.As with other drugs in this class, some isolates of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin tablets. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance
-
2 DOSAGE & ADMINISTRATION2.1 Dosage of Levofloxacin Tablets in Adult Patients with Creatinine Clearance > 50mL/minute - The usual dose of Levofloxacin Tablets is 250 mg, 500 mg, or 750 mg administered orally every 24 ...
2.1 Dosage of Levofloxacin Tablets in Adult Patients with Creatinine Clearance > 50mL/minute
The usual dose of Levofloxacin Tablets is 250 mg, 500 mg, or 750 mg administered orally every 24 hours, as indicated by infection and described in Table 1.
These recommendations apply to patients with creatinine clearance ≥ 50 mL/minute. For patients with creatinine clearance less than 50 mL/min, adjustments to the dosing regimen are required [see Dosage and Administration (2.3)].
Table 1: Dosage of Levofloxacin Tablets in Adult Patients with Creatinine Clearance greater than or equal to 50 mL/minute)
Type of Infection*
Dosed Every 24 hours
Duration (days)†
Nosocomial Pneumonia
750 mg
7 to 14
Community Acquired Pneumonia‡
500 mg‡
7 to 14‡
Community Acquired Pneumonia§
750 mg§
5§
Complicated Skin and Skin Structure Infections (SSSI)
750 mg
7 to 14
Uncomplicated SSSI
500 mg
7 to 10
Chronic Bacterial Prostatitis
500 mg
28
Inhalational Anthrax (Post-Exposure), adult and pediatric patients weighing 50 kg Þ,ß or greater
Pediatric patients weighing 30 kg to less than 50 kg Þ,ß
500 mg
see Table 2 below (2.2)
60ß
60ß
Plague, adult and pediatric patients weighing 50 kg à or greater Pediatric patients weighing 30 kg to less than 50 kg
500 mg
see Table 2 below (2.2)
10 to 14
10 to 14
Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP)¶
750 mg
5
Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP)#
250 mg#
10#
Uncomplicated Urinary Tract Infection
250 mg
3
Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB)
500 mg
7
Acute Bacterial Sinusitis (ABS)
750 mg
5
500 mg
10 to 14
* Due to the designated pathogens [see Indications and Usage (1)].
† Sequential therapy (intravenous levofloxacin to oral levofloxacin tablets) may be instituted at the discretion of the healthcare provider.
‡ Due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae [see Indications and Usage (1.2)].§ Due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, or Chlamydophila pneumoniae [see Indications and Usage (1.3)].
¶ This regimen is indicated for cUTI due to Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and AP due to E. coli, including cases with concurrent bacteremia.
# This regimen is indicated for cUTI due to Enterococcus faecalis, Enterococcus cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa; and for AP due to E. coli.
Þ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized B. anthracis. This indication is based on a surrogate endpoint. Levofloxacin plasma concentrations achieved in humans are reasonably likely to predict clinical benefit [see Clinical Studies (14.9)].
ß The safety of levofloxacin tablets in adults for durations of therapy beyond 28 days or in pediatric patients for durations beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients [see Warnings and Precautions (5.12), Use in Specific Populations (8.4), and Clinical Studies (14.9)]. Prolonged levofloxacin tablets therapy should only be used when the benefit outweighs the risk.
à Drug administration should begin as soon as possible after suspected or confirmed exposure to Yersinia pestis. Higher doses of levofloxacin tablets typically used for treatment of pneumonia can be used for treatment of plague, if clinically indicated.
2.2 Dosage of Levofloxacin Tablets in Pediatric Patients with Inhalational Anthrax or Plague
The dosage of levofloxacin tablets for inhalational anthrax (post-exposure) and plague in pediatric patients who weigh 30 kg or greater is described below in Table 2. Levofloxacin Tablets cannot be administered to patients who weigh less than 30 kg because of the limitations of the available strength. Alternative formulations of levofloxacin may be considered for pediatric patients who weigh less than 30 kg.
Table 2: Levofloxacin Tablets Dosage in Pediatric Patients Weighing 30 kg or greater with Inhalational Anthrax (Post-Exposure) and Plague*Type of Infection*
Dose
Frequency
Duration†
Inhalational Anthrax (post-exposure)‡,§
Pediatric patients weighing 50 kg or greater
500 mg
every 24 hours
60 days§
Pediatric patients weighing 30 kg to less than 50kg
250 mg
every 12 hours
60 days§
Plague¶
Pediatric patients weighing 50 kg or greater
500 mg
every 24 hours
10 to 14 days
Pediatric patients weighing 30 kg to less than 50 kg
250 mg
every 12 hours
10 to 14 days
* Due to Bacillus anthracis [see Indications and Usage (1.13)] and Yersinia pestis [see Indications and Usage (1.14)].
† Sequential therapy (intravenous levofloxacin injection to oral levofloxacin tablets) may be instituted at the discretion of the healthcare provider.
‡ Begin levofloxacin tablets as soon as possible after suspected or confirmed exposure to aerosolized B. anthracis.
§ The safety of levofloxacin tablets in pediatric patients for durations of therapy beyond 14 days has not been studied. [see Warnings and Precautions (5.12), Use in Specific Populations (8.4), and Clinical Studies (14.9)]. Begin levofloxacin tablets as soon as possible after suspected or confirmed exposure to Yersinia pestis.2.3 Dosage Adjustment in Adults with Renal Impairment
Administer levofloxacin with caution in patients with renal impairment. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced in these patients.
In patients with renal impairment (creatinine clearance less than 50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance [see Use in Specific Populations (8.6)]. No adjustment is necessary for patients with a creatinine clearance greater than or equal to 50mL/minute
Table 3 shows how to adjust dose based on creatinine clearance.
Table 3: Dosage Adjustment in Adult Patients with Renal Impairment (Creatinine Clearance less than 50 mL/minute)
Creatinine Clearance greater than or equal to 50 mL/ minute Creatinine Clearance
20 to 49 mL/minuteCreatinine Clearance
10 to 19 mL/minuteHemodialysis or Chronic Ambulatory Peritoneal Dialysis (CAPD) 750 mg every 24 hours
750 mg every 48 hours
750 mg initial dose, then
500 mg every 48 hours
750 mg initial dose, then
500 mg every 48 hours500 mg every 24 hours
500 mg initial dose, then
250 mg every 24 hours
500 mg initial dose, then
250 mg every 48 hours
500 mg initial dose, then
250 mg every 48 hours250 mg every 24 hours
No dosage adjustment required
250 mg every 48 hours.
If treating uncomplicated UTI, then no dosage adjustment is requiredNo information on dosing adjustment is available
2.4 Drug Interaction With Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
Levofloxacin Tablets should be administered at least two hours before or two hours after antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine chewable/buffered tablets or the pediatric powder for oral solution [see Drug Interactions (7.1) and Patient Counseling Information (17)].
2.5 Important Administration Instructions
Levofloxacin can be administered without regard to food.
If patients miss a dose, they should take it as soon as possible anytime up to 8 hours prior to their next scheduled dose. If less than 8 hours remain before the next dose, wait until their next scheduled dose.
Close2.6 Hydration for Patients Receiving Levofloxacin Tablets
Adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of highly concentrated urine. Crystalluria and cylindruria have been reported with quinolones [see Adverse Reactions (6.1) and Patient Counseling Information (17)].
-
3 DOSAGE FORMS & STRENGTHSLevofloxacin Tablets, USP - 250 mg pink colored, capsule shaped, biconvex, film coated tablets, debossed 'T4' on one side and plain on other side - 500 mg peach colored, capsule shaped, biconvex ...
Levofloxacin Tablets, USP
- 250 mg pink colored, capsule shaped, biconvex, film coated tablets, debossed 'T4' on one side and plain on other side
- 500 mg peach colored, capsule shaped, biconvex, film coated tablets, debossed 'T7' on one side and plain on other side
- 750 mg white colored, capsule shaped, biconvex, film coated tablets, debossed 'T5' on one side and plain on other side
-
4 CONTRAINDICATIONSLevofloxacin tablets are contraindicated in persons with known hypersensitivity to levofloxacin, or other quinolone antibacterials [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects - Fluoroquinolones ...
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects
Fluoroquinolones, including levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting levofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see Warnings and Precautions (5.2, 5.3, 5.4)].
Discontinue levofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including levofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
5.2 Tendinitis and Tendon Rupture
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)]. This adverse reaction most frequently involves the Achilles tendon and has also been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites. Tendinitis or tendon rupture can occur within hours or days of starting levofloxacin or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally.
The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Discontinue levofloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Avoid levofloxacin in patients who have a history of tendon disorders or tendon rupture [see Adverse Reactions (6.3)and Patient Counseling Information (17)].
5.3 Peripheral Neuropathy
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including levofloxacin. Symptoms may occur soon after initiation of levofloxacin and may be irreversible in some patients [see Warnings and Precautions (5.1) and Adverse Reactions (6.1, 6.2)].
Discontinue levofloxacin immediately if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation. Avoid fluoroquinolones, including levofloxacin, in patients who have previously experienced peripheral neuropathy [see Adverse Reactions (6) and Patient Counseling Information (17)].
5.4 Central Nervous System Effects
Psychiatric Adverse Reactions
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of psychiatric adverse reactions, including: toxic psychoses, hallucinations, or paranoia; depression, or suicidal thoughts; anxiety, agitation, restlessness, or nervousness; confusion, delirium, disorientation, or disturbances in attention; insomnia or nightmares; memory impairment. Attempted or completed suicide have been reported, especially in patients with a medical history of depression, or an underlying risk factor for depression. These reactions may occur following the first dose. If these reactions occur in patients receiving levofloxacin, discontinue levofloxacin and institute appropriate measures.
Central Nervous System Adverse Reactions
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (including pseudotumor cerebri), tremors, and lightheadedness. As with other fluoroquinolones levofloxacin should be used with caution in patients with a known or suspected central nervous system (CNS) disorder that may predispose them to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose them to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). If these reactions occur in patients receiving levofloxacin, discontinue levofloxacin and institute appropriate measures [see Adverse Reactions (6), Drug Interactions (7.4, 7.5), and Patient Counseling Information (17)].5.5 Exacerbation of Myasthenia Gravis
Fluoroquinolones, including levofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Postmarketing serious adverse reactions, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in patients with myasthenia gravis. Avoid levofloxacin in patients with a known history of myasthenia gravis [see Adverse Reactions (6.3) and Patient Counseling Information (17)].
5.6 Other Serious and Sometimes Fatal Adverse Reactions
Other serious and sometimes fatal adverse reactions, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with fluoroquinolones, including levofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
Discontinue levofloxacin immediately at the first appearance of skin rash, jaundice, or any other sign of hypersensitivity and institute supportive measures [see Adverse Reactions (6) and Patient Counseling Information (17)].
5.7 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with fluoroquinolones, including levofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat, or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. Levofloxacin should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated [see Adverse Reactions (6) and Patient Counseling Information (17) ].
5.8 Hepatotoxicity
Post-marketing reports of severe hepatotoxicity (including acute hepatitis and fatal events) have been received for patients treated with levofloxacin. No evidence of serious drug-associated hepatotoxicity was detected in clinical trials of over 7,000 patients. Severe hepatotoxicity generally occurred within 14 days of initiation of therapy and most cases occurred within 6 days. Most cases of severe hepatotoxicity were not associated with hypersensitivity [see Warnings and Precautions (5.6)]. The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. Levofloxacin should be discontinued immediately if the patient develops signs and symptoms of hepatitis [see Adverse Reactions (6) and Patient Counseling Information (17)].
5.9 Risk of Aortic Aneurysm and Dissection
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients. The cause for the increased risk has not been identified. In patients with a known aortic aneurysm or patients who are at greater risk for aortic aneurysms, reserve levofloxacin for use only when there are no alternative antibacterial treatments available.
5.10 Clostridium difficile- Associated Diarrhea
Clostridium difficile-associated
diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including levofloxacin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse Reactions (6.2) and Patient Counseling Information (17)].5.11 Prolongation of the QT Interval
Some fluoroquinolones, including levofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving fluoroquinolones, including levofloxacin. Levofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval [see Adverse Reactions (6.3), Use in Specific Populations (8.5), and Patient Counseling Information (17)].
5.12 Musculoskeletal Disorders in Pediatric Patients and Arthropathic Effects in Animals
Levofloxacin is indicated in pediatric patients (6 months of age and older) only for the prevention of inhalational anthrax (post-exposure) and for plague [see Indications and Usage (1.7, 1.8)]. An increased incidence of musculoskeletal disorders (arthralgia, arthritis, tendinopathy, and gait abnormality) compared to controls has been observed in pediatric patients receiving levofloxacin [see Use in Specific Populations (8.4)].
In immature rats and dogs, the oral and intravenous administration of levofloxacin resulted in increased osteochondrosis. Histopathological examination of the weight-bearing joints of immature dogs dosed with levofloxacin revealed persistent lesions of the cartilage. Other fluoroquinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species [see Animal Toxicology and/or Pharmacology (13.2) ].5.13 Blood Glucose Disturbances
Fluoroquinolones, including levofloxacin, have been associated with disturbances of blood glucose, including symptomatic hyperglycemia and hypoglycemia, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. Severe cases of hypoglycemia resulting in coma or death have been reported. If a hypoglycemic reaction occurs in a patient being treated with levofloxacin, discontinue levofloxacin and initiate appropriate therapy immediately [see Adverse Reactions (6.2), Drug Interactions (7.3) and Patient Counseling Information (17)].
5.14 Photosensitivity/ Phototoxicity
Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (e.g., burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of fluoroquinolones after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if photosensitivity/phototoxicity occurs [see Adverse Reactions (6.3) and Patient Counseling Information (17)].
Close5.15 Development of Drug Resistant Bacteria
Prescribing levofloxacin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria [see Patient Counseling Information (17)].
-
6 ADVERSE REACTIONSThe following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling: Disabling and Potentially Irreversible Serious Adverse ...
The following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling:
- Disabling and Potentially Irreversible Serious Adverse Reactions [see Warnings and Precautions (5.1)]
- Tendinitis and Tendon Rupture [see Warnings and Precautions (5.2)]
- Peripheral Neuropathy [see Warnings and Precautions (5.3)]
- Central Nervous System Effects [see Warnings and Precautions (5.4)]
- Exacerbation of Myasthenia Gravis [see Warnings and Precautions (5.5)]
- Other Serious and Sometimes Fatal Reactions [see Warnings and Precautions (5.6)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
- Hepatotoxicity [see Warnings and Precautions (5.8)]
- Risk of Aortic Aneurysm and Dissection [see Warnings and Precautions (5.9)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.10)]
- Prolongation of the QT Interval [see Warnings and Precautions (5.11)]
- Musculoskeletal Disorders in Pediatric Patients [see Warnings and Precautions (5.12)]
- Blood Glucose Disturbances [see Warnings and Precautions (5.13)]
- Photosensitivity/Phototoxicity [see Warnings and Precautions (5.14)]
- Development of Drug Resistant Bacteria [see Warnings and Precautions (5.15)]
Crystalluria and cylindruria have been reported with quinolones, including levofloxacin. Therefore, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of a highly concentrated urine [see Dosage and Administration (2.5)].
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to levofloxacin in 7537 patients in 29 pooled Phase 3 clinical trials. The population studied had a mean age of 50 years (approximately 74% of the population was < 65 years of age), 50% were male, 71% were Caucasian, 19% were Black. Patients were treated with levofloxacin for a wide variety of infectious diseases [see Indications and Usage (1)]. Patients received levofloxacin doses of 750 mg once daily, 250 mg once daily, or 500 mg once or twice daily. Treatment duration was usually 3–14 days, and the mean number of days on therapy was 10 days.
The overall incidence, type and distribution of adverse reactions was similar in patients receiving levofloxacin doses of 750 mg once daily, 250 mg once daily, and 500 mg once or twice daily. Discontinuation of levofloxacin due to adverse drug reactions occurred in 4.3% of patients overall, 3.8% of patients treated with the 250 mg and 500 mg doses and 5.4% of patients treated with the 750 mg dose. The most common adverse drug reactions leading to discontinuation with the 250 and 500 mg doses were gastrointestinal (1.4%), primarily nausea (0.6%); vomiting (0.4%); dizziness (0.3%); and headache (0.2%). The most common adverse drug reactions leading to discontinuation with the 750 mg dose were gastrointestinal (1.2%), primarily nausea (0.6%), vomiting (0.5%); dizziness (0.3%); and headache (0.3%).
Adverse reactions occurring in ≥1% of levofloxacin -treated patients and less common adverse reactions, occurring in 0.1 to <1% of levofloxacin -treated patients, are shown in Table 4 and Table 5, respectively. The most common adverse drug reactions (≥3%) are nausea, headache, diarrhea, insomnia, constipation, and dizziness.
Table 4: Common (≥1%) Adverse Reactions Reported in Clinical Trials with Levofloxacin# System/Organ ClassSystem/Organ Class Adverse ReactionAdverse Reaction %
(N=7537)%
(N=7537)System/Organ Class Adverse Reaction %
(N=7537)
Infections and Infestations
moniliasis
1
Psychiatric Disorders
insomnia*[see Warnings and Precautions (5.4)]
4
Nervous System Disorders
headache
dizziness [see Warnings and Precautions (5.4)]
6
3
Respiratory, Thoracic and Mediastinal Disorders
dyspnea [see Warnings and Precautions (5.7)]
1
Gastrointestinal Disorders
nausea
diarrhea
constipation
abdominal pain
vomiting
dyspepsia
7
5
3
2
2
2
Skin and Subcutaneous Tissue Disorders
rash [see Warnings and Precautions (5.7)]
pruritus
2
1
Reproductive System and Breast Disorders
Vaginitis
1†
General Disorders and Administration Site Conditions
edema
injection site reaction
chest pain
1
1
1
# pool of studies included IV and oral administration
Table 5: Less Common (0.1 to 1%) Adverse Reactions Reported in Clinical Trials with Levofloxacin (N=7537) System/Organ ClassSystem/Organ Class Adverse ReactionAdverse Reaction System/Organ Class Adverse Reaction Infections and Infestations
genital moniliasis
Blood and Lymphatic System Disorders
anemia
thrombocytopenia
granulocytopenia
[see Warnings and Precautions (5.6)]
Immune System Disorders
allergic reaction [See Warnings and Precautions (5.6,5.7)]
Metabolism and Nutrition Disorders
hyperglycemia
hypoglycemia
[see Warnings and Precautions (5.13)]
hyperkalemia
Psychiatric Disorders
anxiety
agitation
confusion
depression
hallucination
nightmare*
[see Warnings and Precautions (5.4)]
sleep disorder*
anorexia
abnormal dreaming*
Nervous System Disorders
tremor
convulsions
[see Warnings and Precautions (5.4)]
paresthesia [see Warnings and Precautions (5.3)]
vertigo
hypertonia
hyperkinesias
abnormal gait
somnolence*
syncope
Respiratory, Thoracic and Mediastinal Disorders
epistaxis
Cardiac Disorders
cardiac arrest
palpitation
ventricular tachycardia
ventricular arrhythmia
Vascular Disorders
phlebitis
Gastrointestinal Disorders
gastritis
stomatitis
pancreatitis
esophagitis
gastroenteritis
glossitis
pseudomembraneous/ C. difficile colitis [see Warnings and Precautions (5.10)]
Hepatobiliary Disorders
abnormal hepatic function
increased hepatic enzymes
increased alkaline phosphatase
Skin and Subcutaneous Tissue Disorders
urticaria [see Warnings and Precautions (5.7)]
Musculoskeletal and Connective Tissue Disorders
arthralgia
tendinitis
[see Warnings and Precautions (5.2)]
myalgia
skeletal pain
Renal and Urinary Disorders
abnormal renal function
acute renal failure [see Warnings and Precautions (5.6)]
*N=7274
In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with quinolones, including levofloxacin. The relationship of the drugs to these events is not presently established.
Close6.2 Postmarketing Experience
Table 6 lists adverse reactions that have been identified during post-approval use of levofloxacin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 6: Postmarketing Reports Of Adverse Drug Reactions
System/Organ ClassSystem/Organ Class Adverse ReactionAdverse Reaction System/Organ Class Adverse Reaction Blood and Lymphatic System Disorders
pancytopenia
aplastic anemia
leukopenia
hemolytic anemia
[see Warnings and Precautions (5.6)]
eosinophilia
Immune System Disorders
hypersensitivity reactions, sometimes fatal including:
anaphylactic/anaphylactoid reactions
anaphylactic shock
angioneurotic edema
serum sickness
[see Warnings and Precautions (5.6,5.7)]
Psychiatric Disorders
psychosis
paranoia
isolated reports of suicidal ideation, suicide attempt and completed suicide
[see Warnings and Precautions (5.4)]
Nervous System Disorders
exacerbation of myasthenia gravis [see Warnings and Precautions (5.5)]
anosmia
ageusia
parosmia
dysgeusia
peripheral neuropathy (may be irreversible) [see Warnings and Precautions (5.3)]
isolated reports of encephalopathy
abnormal electroencephalogram (EEG)
dysphonia
pseudotumor cerebri [see Warnings and Precautions (5.4)]
Eye Disorders
uveitis
vision disturbance, including diplopia
visual acuity reduced
vision blurred
scotoma
Ear and Labyrinth Disorders
hypoacusis
tinnitus
Cardiac Disorders
isolated reports of torsade de pointes
electrocardiogram QT prolonged
[see Warnings and Precautions (5.11)]
tachycardia
Vascular Disorders
vasodilatation
Respiratory, Thoracic and Mediastinal Disorders
isolated reports of allergic pneumonitis [see Warnings and Precautions (5.6)]
Hepatobiliary Disorders
hepatic failure (including fatal cases)
hepatitis
jaundice
[see Warnings and Precautions 5. 6,5.8)]
Skin and Subcutaneous Tissue Disorders
bullous eruptions to include:
Stevens-Johnson Syndrome
toxic epidermal necrolysis
Acute Generalized Exanthematous Pustulosis (AGEP)
fixed drug eruptions
erythema multiforme
[see Warnings and Precautions (5.6)]
photosensitivity/phototoxicity reaction [see Warnings and Precautions (5.14)]
leukocytoclastic vasculitis
Musculoskeletal and Connective Tissue Disorders
tendon rupture [see Warnings and Precautions (5.2)]
muscle injury, including rupture
rhabdomyolysis
Renal and Urinary Disorders
interstitial nephritis [see Warnings and Precautions (5.6)]
General Disorders and Administration Site Conditions
multi-organ failure
pyrexia
Investigations
prothrombin time prolonged
international normalized ratio prolonged
muscle enzymes increased
-
7 DRUG INTERACTIONS7.1 Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins - While the chelation by divalent cations is less marked than with other fluoroquinolones, concurrent administration of ...
7.1 Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
While the chelation by divalent cations is less marked than with other fluoroquinolones, concurrent administration of levofloxacin tablets with antacids containing magnesium, or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. Tablets with antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine may substantially interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least two hours before or two hours after oral levofloxacin administration.
7.2 Warfarin
No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for R- and S- warfarin was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of warfarin on levofloxacin absorption and disposition was observed. However, there have been reports during the postmarketing experience in patients that levofloxacin enhances the effects of warfarin. Elevations of the prothrombin time in the setting of concurrent warfarin and levofloxacin use have been associated with episodes of bleeding. Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if levofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding [see Adverse Reactions (6.3) andPatient Counseling Information (17)].
7.3 Antidiabetic Agents
Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with fluoroquinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered [see Warnings and Precautions (5.13); Adverse Reactions (6.2) and Patient Counseling Information (17)]
7.4 Non-Steroidal Anti-Inflammatory Drugs
The concomitant administration of a non-steroidal anti-inflammatory drug with a fluoroquinolone, including levofloxacin, may increase the risk of CNS stimulation and convulsive seizures [see Warnings and Precautions (5.4)].
7.5 Theophylline
No significant effect of levofloxacin on the plasma concentrations, AUC, and other disposition parameters for theophylline was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of theophylline on levofloxacin absorption and disposition was observed. However, concomitant administration of other fluoroquinolones with theophylline has resulted in prolonged elimination half-life, elevated serum theophylline levels, and a subsequent increase in the risk of theophylline-related adverse reactions in the patient population. Therefore, theophylline levels should be closely monitored and appropriate dosage adjustments made when levofloxacin is co-administered. Adverse reactions, including seizures, may occur with or without an elevation in serum theophylline levels [see Warnings and Precautions (5.4)].
7.6 Cyclosporine
No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for cyclosporine was detected in a clinical study involving healthy volunteers. However, elevated serum levels of cyclosporine have been reported in the patient population when co-administered with some other fluoroquinolones. Levofloxacin Cmax and ke were slightly lower while Tmax and t½ were slightly longer in the presence of cyclosporine than those observed in other studies without concomitant medication. The differences, however, are not considered to be clinically significant. Therefore, no dosage adjustment is required for levofloxacin or cyclosporine when administered concomitantly.
7.7 Digoxin
No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for digoxin was detected in a clinical study involving healthy volunteers. Levofloxacin absorption and disposition kinetics were similar in the presence or absence of digoxin. Therefore, no dosage adjustment for levofloxacin or digoxin is required when administered concomitantly.
7.8 Probenecid and Cimetidine
No significant effect of probenecid or cimetidine on the Cmax of levofloxacin was observed in a clinical study involving healthy volunteers. The AUC and t½ of levofloxacin were higher while CL/F and CLR were lower during concomitant treatment of levofloxacin with probenecid or cimetidine compared to levofloxacin alone. However, these changes do not warrant dosage adjustment for levofloxacin when probenecid or cimetidine is co-administered.
Close7.9 Interactions with Laboratory or Diagnostic Testing
Some fluoroquinolones, including levofloxacin, may produce false-positive urine screening results for opiates using commercially available immunoassay kits. Confirmation of positive opiate screens by more specific methods may be necessary.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published information from case reports, case control studies and observational studies on levofloxacin administered during pregnancy have not identified any ...
8.1 Pregnancy
Risk Summary
Published information from case reports, case control studies and observational studies on levofloxacin administered during pregnancy have not identified any drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal reproduction studies, oral administration of levofloxacin to pregnant rats and rabbits during organogenesis at doses up to 9.4 times and 1.1 times the maximum recommended human dose (MRHD), respectively, did not result in teratogenicity. Fetal toxicity was seen in the rat study, but was absent at doses up to 1.2 times the maximum recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Levofloxacin was not teratogenic in an embryofetal development study in rats treated during organogenesis with oral doses as high as 810 mg/kg/day which corresponds to 9.4 times the MRHD (based upon doses normalized for total body surface area). The oral dose of 810 mg/kg/day (high dose) to rats caused decreased fetal body weight and increased fetal mortality that was not seen at the next lower dose (mid-dose, 90 mg/kg/day, equivalent to 1.2 times the MRHD (based upon doses normalized for total body surface area).
Maternal toxicity was limited to lower weight gain in the mid and high dose groups. No teratogenicity was observed in an embryofetal development study in rabbits dosed orally during organogenesis with doses as high as 50 mg/kg/day, which corresponds to 1.1 times the MRHD (based upon doses normalized for total body surface area). Maternal toxicity at that dose consisted of lower weight gain and decreased food consumption relative to controls and abortion in four of sixteen dams.
8.2 Lactation
Risk Summary
Published literature reports that levofloxacin is present in human milk following intravenous and oral administration (see Data). There is no information regarding effects of levofloxacin on milk production or the breastfed infant. Because of the potential risks of serious adverse reactions, in breastfed infants, for most indications, a lactating woman may consider pumping and discarding breast milk during treatment with levofloxacin and an additional two days (five half-lives) after the last dose. Alternatively, advise a lactating woman that breastfeeding is not recommended during treatment with levofloxacin and for an additional two days (five half-lives) after the last dose [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
However, for inhalation anthrax (post exposure), during an incident resulting in exposure to anthrax, the risk-benefit assessment of continuing breastfeeding while the mother (and potentially the infant) is (are) on levofloxacin may be acceptable [see Dosage and Administration (2.2), Pediatric Use (8.4), and Clinical Studies (14.2)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levofloxacin and any potential adverse effects on the breastfed child from levofloxacin or from the underlying maternal condition.
Data
A published literature reports that peak levofloxacin human milk concentration was 8.2 mg/L at 5 hours after dosing in a woman who received 500 mg of intravenous, followed by oral, levofloxacin daily. For an infant fed exclusively with human milk (approximately 900 ml/day), an estimated maximum daily dose of levofloxacin through breastfeeding is 5 mg (i.e., approximately 1% of maternal daily dose). The above data come from a single case and may not be generalizable to the general population of lactating women.
8.4 Pediatric Use
Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species. [see Warnings and Precautions [5.12] and Animal Toxicology and/or Pharmacology (13.2)]
Inhalational Anthrax (Post-Exposure)
Levofloxacin is indicated in pediatric patients 6 months of age and older, for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate. The safety of levofloxacin in pediatric patients treated for more than 14 days has not been studied [see Indications and Usage (1.7), Dosage and Administration (2.2) andClinical Studies (14.9)].
Plague
Levofloxacin is indicated in pediatric patients, 6 months of age and older, for treatment of plague, including pneumonic and septicemic plague due to Yersinia pestis (Y. pestis) and prophylaxis for plague. Efficacy studies of levofloxacin could not be conducted in humans with pneumonic plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals. The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate [see Indications and Usage (1.8), Dosage and Administration (2.2) and Clinical Studies (14.10)].
Safety and effectiveness of levofloxacin tablets in pediatric patients below the age of six months have not been established.
Pharmacokinetics following intravenous administration
The pharmacokinetics of levofloxacin following a single intravenous dose were investigated in pediatric patients ranging in age from six months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients resulting in lower plasma exposures than adults for a given mg/kg dose [see Clinical Pharmacology (12.3) and Clinical Studies (14.9)].Dosage in Pediatric Patients with Inhalational Anthrax or Plague
For the recommended levofloxacin tablet dosage in pediatric patients with inhalational anthrax or plague, see Dosage and Administration (2.2). Levofloxacin Tablets cannot be administered to pediatric patients who weigh less than 30 kg because of the limitations of the available strengths. Alternative formulations of levofloxacin may be considered for pediatric patients who weigh less than 30 kg.
Adverse Reactions
In clinical trials, 1534 pediatric patients (6 months to 16 years of age) were treated with oral and intravenous levofloxacin. Pediatric patients 6 months to 5 years of age received levofloxacin 10 mg/kg twice a day and pediatric patients greater than 5 years of age received 10 mg/kg once a day (maximum 500 mg per day) for approximately 10 days. Levofloxacin tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)].
A subset of pediatric patients in the clinical trials (1340 levofloxacin-treated and 893 non-fluoroquinolone-treated) enrolled in a prospective, long-term surveillance study to assess the incidence of protocol-defined musculoskeletal disorders (arthralgia, arthritis, tendinopathy, gait abnormality) during 60 days and 1 year following the first dose of the study drug. Pediatric patients treated with levofloxacin had a significantly higher incidence of musculoskeletal disorders when compared to the non-fluoroquinolone-treated children as illustrated in Table 7. Levofloxacin tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)].
Table 7: Incidence of Musculoskeletal Disorders in Pediatric Clinical Trial
Follow-up Period Levofloxacin
N = 1340Non-Fluoroquinolone*
N = 893p-value† 60 days 28 (2.1%) 8 (0.9%) p = 0.038 1 year‡ 46 (3.4%) 16 (1.8%) p = 0.025 * Non-Fluoroquinolone: ceftriaxone, amoxicillin/clavulanate, clarithromycin
† 2-sided Fisher’s Exact Test
‡
There were 1199 levofloxacin-treated and 804 non-fluoroquinolone-treated pediatric patients who had a one-year evaluation visit. However, the incidence of musculoskeletal disorders was calculated using all reported events during the specified period for all pediatric patients enrolled regardless of whether they completed the 1-year evaluation visit.
Arthralgia was the most frequently occurring musculoskeletal disorder in both treatment groups. Most of the musculoskeletal disorders in both groups involved multiple weight-bearing joints. Disorders were moderate in 8/46 (17%) children and mild in 35/46 (76%) levofloxacin-treated pediatric patients and most were treated with analgesics. The median time to resolution was 7 days for levofloxacin -treated pediatric patients and 9 for non-fluoroquinolone-treated children (approximately 80% resolved within 2 months in both groups). No pediatric patient had a severe or serious disorder and all musculoskeletal disorders resolved without sequelae.
Vomiting and diarrhea were the most frequently reported adverse reactions, occurring in similar frequency in the levofloxacin -treated and non-fluoroquinolone-treated pediatric patients.
In addition to the adverse reactions reported in pediatric patients in clinical trials, adverse reactions reported in adults during clinical trials or post-marketing experience [see Adverse Reactions (6)] may also be expected to occur in pediatric patients.8.5 Geriatric Use
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as levofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing levofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue levofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur [see Boxed Warning; Warnings and Precautions (5.2); and Adverse Reactions (6.3)].
In phase 3 clinical trials, 1,945 levofloxacin -treated patients (26%) were ≥ 65 years of age. Of these, 1,081 patients (14%) were between the ages of 65 and 74 and 864 patients (12%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Severe, and sometimes fatal, cases of hepatotoxicity have been reported post-marketing in association with levofloxacin. The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. Levofloxacin should be discontinued immediately if the patient develops signs and symptoms of hepatitis [see Warnings and Precautions (5.8)].
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients. [see Warnings and Precautions (5.9)].Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using levofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g., known QT prolongation, uncorrected hypokalemia) [see Warnings and Precautions (5.11)].
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in patients with renal impairment (creatinine clearance < 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of levofloxacin are not required following hemodialysis or CAPD [see Dosage and Administration (2.3)].
Close8.7 Hepatic Impairment
Pharmacokinetic studies in patients with hepatic impairment have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment.
-
10 OVERDOSAGEIn the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or ...
In the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Levofloxacin exhibits a low potential for acute toxicity. Mice, rats, dogs and monkeys exhibited the following clinical signs after receiving a single high dose of levofloxacin: ataxia, ptosis, decreased locomotor activity, dyspnea, prostration, tremors, and convulsions. Doses in excess of 1500 mg/kg orally (approximately 10 or 19 times MRHD in mice and rats, respectively) and 250 mg/kg IV produced significant mortality (estimated to be greater than or equal to 50%) in rodents.
Close -
11 DESCRIPTIONLevofloxacin USP are synthetic antibacterial agents for oral administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug ...
Levofloxacin USP are synthetic antibacterial agents for oral administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (--(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate.
Figure 1: The Chemical Structure of Levofloxacin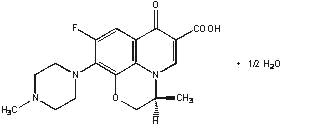
The empirical formula is C18H20FN3O4 •½ H2O and the molecular weight is 370.38. Levofloxacin USP is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine.
The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin, USP is essentially constant (approximately 100 mg/mL). Levofloxacin USP is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9.
Levofloxacin USP has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al +3 >Cu +2 >Zn +2 >Mg +2 >Ca +2 .
Levofloxacin USP is available as film-coated tablets and contain the following inactive ingredients:- 250 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red iron oxide.
- 500 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red and yellow iron oxides.
- 750 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80.
USP dissolution test 4.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Levofloxacin is a member of the fluoroquinolone class of antibacterial agents [see Microbiology(12.4)]. 12.3 Pharmacokinetics - The mean ±SD pharmacokinetic ...
12.1 Mechanism of Action
Levofloxacin is a member of the fluoroquinolone class of antibacterial agents [see Microbiology(12.4)].
12.3 Pharmacokinetics
The mean ±SD pharmacokinetic parameters of levofloxacin determined under single and steady-state conditions following administration of the oral tablets, are summarized in Table 8.
Table 8: Mean ±SD Levofloxacin PK Parameters
Regimen
Cmax (mcg/mL)
Tmax (h)
AUC (mcg·h/mL)
CL/F1 (mL/min)
Vd/F2 (L)
t 1/2 (h)
CLR (mL/
min)
Single dose
250 mg oral tablet3
2.8 ± 0.4
1.6 ± 1.0
27.2 ± 3.9
156 ± 20
ND
7.3 ± 0.9
142 ± 21
500 mg oral tablet3*
5.1 ± 0.8
1.3 ± 0.6
47.9 ± 6.8
178 ± 28
ND
6.3 ± 0.6
103 ± 30
750 mg oral tablet4*
9.3 ± 1.6
1.6 ± 0.8
101 ± 20
129 ± 24
83 ± 17
7.5 ± 0.9
ND
Multiple dose
500 mg every 24h oral tablet3
5.7 ± 1.4
1.1 ± 0.4
47.5 ± 6.7
175 ± 25
102 ± 22
7.6 ± 1.6
116 ± 31
750 mg every 24h oral tablet4
8.6 ± 1.9
1.4 ± 0.5
90.7 ± 17.6
143 ± 29
100 ± 16
8.8 ± 1.5
116 ± 28
500 mg oral tablet single dose, effects of gender and age:
Male5
5.5 ± 1.1
1.2 ± 0.4
54.4 ± 18.9
166 ± 44
89 ± 13
7.5 ± 2.1
126 ± 38
Female6
7.0 ± 1.6
1.7 ± 0.5
67.7 ± 24.2
136 ± 44
62 ± 16
6.1 ± 0.8
106 ± 40
Young7
5.5 ± 1.0
1.5 ± 0.6
47.5 ± 9.8
182 ± 35
83 ± 18
6.0 ± 0.9
140 ± 33
Elderly8
7.0 ± 1.6
1.4 ± 0.5
74.7 ± 23.3
121 ± 33
67 ± 19
7.6 ± 2.0
91 ± 29
500 mg oral single dose tablet, patients with renal impairment:
CLCR 50–80 mL/min
7.5 ± 1.8
1.5 ± 0.5
95.6 ± 11.8
88 ± 10
ND
9.1 ± 0.9
57 ± 8
CLCR 20–49 mL/min
7.1 ± 3.1
2.1 ± 1.3
182.1 ± 62.6
51 ± 19
ND
27 ± 10
26 ± 13
CLCR <20 mL/min
8.2 ± 2.6
1.1 ± 1.0
263.5 ± 72.5
33 ± 8
ND
35 ± 5
13 ± 3
Hemodialysis
5.7 ± 1.0
2.8 ± 2.2
ND
ND
ND
76 ± 42
ND
CAPD
6.9 ± 2.3
1.4 ± 1.1
ND
ND
ND
51 ± 24
ND
1 clearance /bioavailability
2 volume of distribution/bioavailability
3 healthy males 18–53 years of age
4 healthy male and female subjects 18–54 years of age
5 healthy males 22–75 years of age
6 healthy females 18–80 years of age
7young healthy male and female subjects 18–36 years of age
8 healthy elderly male and female subjects 66–80 years of age
*Absolute bioavailability; F=0.99 ± 0.08 from a 500 mg tablet and F=0.99 ± 0.06 from a 750 mg tablet; ND=not determined.Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral or IV dosing regimens. Steady-state conditions are reached within 48 hours following a 500 mg or 750 mg once-daily dosage regimen. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily oral dosage regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 mcg/mL after the 500 mg doses, and 8.6 ± 1.9 and 1.1 ± 0.4 mcg/mL after the 750 mg doses, respectively. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily IV regimens were approximately 6.4 ± 0.8 and 0.6 ± 0.2 mcg/mL after the 500 mg doses, and 12.1 ± 4.1 and 1.3 ± 0.71 mcg/mL after the 750 mg doses, respectively.
Absorption
Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hours after oral dosing. The absolute bioavailability of levofloxacin from a 500 mg tablet and a 750 mg tablet of levofloxacin are both approximately 99%, demonstrating complete oral absorption of levofloxacin. Following a single intravenous dose of levofloxacin to healthy volunteers, the mean ± SD peak plasma concentration attained was 6.2 ± 1.0 mcg/mL after a 500 mg dose infused over 60 minutes and 11.5 ± 4.0 mcg/mL after a 750 mg dose infused over 90 minutes. Oral administration of a 500 mg dose of levofloxacin with food prolongs the time to peak concentration by approximately 1 hour and decreases the peak concentration by approximately 14% following tablet and approximately 25% following oral solution administration. Therefore, levofloxacin tablets can be administered without regard to food.The plasma concentration profile of levofloxacin after IV administration is similar and comparable in extent of exposure (AUC) to that observed for levofloxacin tablets when equal doses (mg/mg) are administered. Therefore, the oral and IV routes of administration can be considered interchangeable.
Distribution
The mean volume of distribution of levofloxacin generally ranges from 74 to 112 L after single and multiple 500 mg or 750 mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissues and in blister fluid of healthy subjects at approximately 3 hours after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple once-daily oral administration of 750 mg and 500 mg doses of levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5- fold higher than plasma concentrations and ranged from approximately 2.4 to 11.3 mcg/g over a 24-hour period after a single 500 mg oral dose.In vitro, over a clinically relevant range (1 to 10 mcg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24 to 38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Elimination
Metabolism
Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hours, whereas less than 4% of the dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion
Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Specific Populations
Geriatric PatientsThere are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects' differences in creatinine clearance are taken into consideration. Following a 500 mg oral dose of levofloxacin to healthy elderly subjects (66 – 80 years of age), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hours, as compared to approximately 6 hours in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. Levofloxacin dose adjustment based on age alone is not necessary [see Use in Specific Populations (8.5)].
Pediatric PatientsThe pharmacokinetics of levofloxacin following a single 7 mg/kg intravenous dose were investigated in pediatric patients ranging in age from 6 months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients, resulting in lower plasma exposures than adults for a given mg/kg dose. Subsequent pharmacokinetic analyses predicted that a dosage regimen of 8 mg/kg every 12 hours (not to exceed 250 mg per dose) for pediatric patients 6 months to 17 years of age would achieve comparable steady state plasma exposures (AUC0-24 and Cmax) to those observed in adult patients administered 500 mg of levofloxacin once every 24 hours. Levofloxacin tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2)]
Male and Female SubjectsThere are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects' differences in creatinine clearance are taken into consideration.
Following a 500 mg oral dose of levofloxacin to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hours, as compared to approximately 6.1 hours in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustment based on gender alone is not necessary.
Racial or Ethnic GroupsThe effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 non-white. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Patients with Renal Impairment
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in adult patients with impaired renal function (creatinine clearance< 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of levofloxacin are not required following hemodialysis or CAPD [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment [See Use in Specific Populations (8.7)]
Patients with Bacterial InfectionThe pharmacokinetics of levofloxacin in patients with serious community-acquired bacterial infections are comparable to those observed in healthy subjects.
Drug-Interaction StudiesThe potential for pharmacokinetic drug interactions between levofloxacin and antacids warfarin, theophylline, cyclosporine, digoxin, probenecid, and cimetidine has been evaluated [see Drug Interactions (7)].
Close12.4 Microbiology
Mechanism of Action
Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination.
Resistance
Fluoroquinolone resistance can arise through mutations in defined regions of DNA gyrase or topoisomerase IV, termed the Quinolone-Resistance Determining Regions (QRDRs), or through altered efflux.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and ⛚-lactam antibiotics, including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10-9 to 10-10). Cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Antimicrobial Activity Levofloxacin has in vitro activity against Gram-negative and Gram-positive bacteria.Levofloxacin has been shown to be active against most isolates of the following bacteria both in vitro and in clinical infections as described in Indications and Usage (1):
Aerobic bacteria
Gram-Positive BacteriaEnterococcus faecalis
Staphylococcus aureus (methicillin-susceptible isolates)Staphylococcus epidermidis (methicillin-susceptible isolates)
Staphylococcus saprophyticus
Streptococcus pneumoniae (including multi-drug resistant isolates [MDRSP]1)
Streptococcus pyogenes
1MDRSP (Multi-drug resistant Streptococcus pneumoniae) isolates are isolates resistant to two or more of the following antibiotics: penicillin (MIC ≥2 mcg/mL), 2nd generation cephalosporins, e.g., cefuroxime; macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
Gram-Negative Bacteria
Enterobacter cloacaeEscherichia coli
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella pneumoniae
Legionella pneumophila
Moraxella catarrhalis
Proteus mirabilis
Pseudomonas aeruginosa
Serratia marcescens
Other microorganisms
Chlamydophila pneumoniae
Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentrations (MIC) less than or equal to the susceptible breakpoint for levofloxacin against isolates of similar genus or organism group. However, efficacy of levofloxacin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic bacteria
Gram-Positive Bacteria
Staphylococcus haemolyticusβ-hemolytic Streptococcus (Group C/F)
β - hemolytic Streptococcus (Group G)
Streptococcus agalactiae
Streptococcus milleri
Viridans group streptococci
Bacillus anthracis
Gram-Negative BacteriaAcinetobacter baumannii
Acinetobacter lwoffii
Bordetella pertussis
Citrobacter koseri
Citrobacter freundii
Enterobacter aerogenes
Enterobacter sakazakii
Klebsiella oxytoca
Morganella morganii
Pantoea agglomerans
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Pseudomonas fluorescens
Yersinia pestis
Anaerobic bacteria
Gram-Positive Bacteria
Clostridium perfringensSusceptibility Tests
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - In a lifetime bioassay in rats, levofloxacin exhibited no carcinogenic potential following daily dietary administration for 2 ...
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
In a lifetime bioassay in rats, levofloxacin exhibited no carcinogenic potential following daily dietary administration for 2 years; the highest dose (100 mg/kg/day) was 1.4 times the Maximum Recommended Human Dose (MRHD) (750 mg) after normalization for total body surface area. Levofloxacin did not shorten the time to tumor development of UV-induced skin tumors in hairless albino (Skh-1) mice at any levofloxacin dose level and was therefore not photo-carcinogenic under conditions of this study. Dermal levofloxacin concentrations in the hairless mice ranged from 25 to 42 mcg/g at the highest levofloxacin dose level (300 mg/kg/day) used in the photo-carcinogenicity study. By comparison, dermal levofloxacin concentrations in human subjects receiving 750 mg of levofloxacin averaged approximately 11.8 mcg/g at Cmax.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay (S. typhimurium and E. coli), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproductive performance in rats at oral doses as high as 360 mg/kg/day, corresponding to 4.2 times the MRHD and intravenous doses as high as 100 mg/kg/day, corresponding to 1.2 times the MRHD after normalization for total body surface area.
Close13.2 Animal Toxicology & or Pharmacology
Levofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested [see Warnings and Precautions (5.12)]. In immature dogs (4–5 months old), oral doses of 10 mg/kg/day for 7 days and intravenous doses of 4 mg/kg/day for 14 days of levofloxacin resulted in arthropathic lesions. Administration at oral doses of 300 mg/kg/day for 7 days and intravenous doses of 60 mg/kg/day for 4 weeks produced arthropathy in juvenile rats. Three-month old beagle dogs dosed orally with levofloxacin at 40 mg/kg/day exhibited clinically severe arthrotoxicity resulting in the termination of dosing at Day 8 of a 14-day dosing routine (dosing was terminated in the low and mid-dose groups on Day 9 due to similar findings at the mid-dose). Slight musculoskeletal clinical effects, in the absence of gross pathological or histopathological effects, resulted from the lowest dose level of 2.5 mg/kg/day (approximately 0.2-fold the pediatric dose based upon AUC comparisons). Synovitis and articular cartilage lesions were observed at the 10 and 40 mg/kg dose levels (approximately 0.7-fold and 2.4-fold the pediatric dose, respectively, based on AUC comparisons). Articular cartilage gross pathology and histopathology persisted to the end of the 18-week recovery period for those dogs from the 10 and 40 mg/kg/day dose levels. The low and mid-dose groups in that study were also evaluated by electron microscopy, revealing compound-related ultrastructural effects in articular cartilage chondrocytes at the end of treatment and at the end of recovery in both of those doses.
When tested in a mouse ear swelling bioassay, levofloxacin exhibited phototoxicity similar in magnitude to ofloxacin, but less phototoxicity than other quinolones.
While crystalluria has been observed in some intravenous rat studies, urinary crystals are not formed in the bladder, being present only after micturition and are not associated with nephrotoxicity.
In mice, the CNS stimulatory effect of quinolones is enhanced by concomitant administration of non-steroidal anti-inflammatory drugs.
In dogs, levofloxacin administered at 6 mg/kg or higher by rapid intravenous injection produced hypotensive effects. These effects were considered to be related to histamine release.
In vitro and in vivo studies in animals indicate that levofloxacin is neither an enzyme inducer nor inhibitor in the human therapeutic plasma concentration range; therefore, no drug metabolizing enzyme-related interactions with other drugs or agents are anticipated. -
14 CLINICAL STUDIES14.1 Nosocomial Pneumonia - Adult patients with clinically and radiologically documented nosocomial pneumonia were enrolled in a multicenter, randomized, open-label study comparing intravenous ...
14.1 Nosocomial Pneumonia
Adult patients with clinically and radiologically documented nosocomial pneumonia were enrolled in a multicenter, randomized, open-label study comparing intravenous levofloxacin (750 mg once daily) followed by oral levofloxacin (750 mg once daily) for a total of 7–15 days to intravenous imipenem/cilastatin (500–1000 mg every 6–8 hours daily) followed by oral ciprofloxacin (750 mg every 12 hours daily) for a total of 7–15 days. Levofloxacin-treated patients received an average of 7 days of intravenous therapy (range: 1–16 days); comparator-treated patients received an average of 8 days of intravenous therapy (range: 1–19 days).
Overall, in the clinically and microbiologically evaluable population, adjunctive therapy was empirically initiated at study entry in 56 of 93 (60.2%) patients in the levofloxacin arm and 53 of 94 (56.4%) patients in the comparator arm. The average duration of adjunctive therapy was 7 days in the levofloxacin arm and 7 days in the comparator. In clinically and microbiologically evaluable patients with documented Pseudomonas aeruginosa infection, 15 of 17 (88.2%) received ceftazidime (N=11) or piperacillin/tazobactam (N=4) in the levofloxacin arm and 16 of 17 (94.1%) received an aminoglycoside in the comparator arm. Overall, in clinically and microbiologically evaluable patients, vancomycin was added to the treatment regimen of 37 of 93 (39.8%) patients in the levofloxacin arm and 28 of 94 (29.8%) patients in the comparator arm for suspected methicillin-resistant S. aureus infection.
Clinical success rates in clinically and microbiologically evaluable patients at the post-therapy visit (primary study endpoint assessed on day 3–15 after completing therapy) were 58.1% for levofloxacin and 60.6% for comparator. The 95% CI for the difference of response rates (levofloxacin minus comparator) was [-17.2, 12.0]. The microbiological eradication rates at the posttherapy visit were 66.7% for levofloxacin and 60.6% for comparator. The 95% CI for the difference of eradication rates (levofloxacin minus comparator) was [-8.3, 20.3]. Clinical success and microbiological eradication rates by pathogen are detailed in Table 9.
Table 9: Clinical Success Rates and Bacteriological Eradication Rates (Nosocomial Pneumonia)
Pathogen N Levofloxacin No. (%) of Patients Microbiologic/Clinical Outcomes N Imipenem/Cilastatin No. (%) of Patients Microbiologic/Clinical Outcomes MSSA* 21
14 (66.7)/13 (61.9)
19
13 (68.4)/15(78.9)
P. aeruginosa† 17
10 (58.8)/11 (64.7)
17
5 (29.4)/7 (41.2)
S. marcescens 11
9 (81.8)/7 (63.6)
7
2 (28.6)/3 (42.9)
E. coli 12
10 (83.3)/7 (58.3)
11
7 (63.6 )/8 (72.7)
K. pneumoniae‡ 11
9 (81.8)/5 (45.5)
7
6 (85.7)/3 (42.9)
H. influenzae 16
13 (81.3)/10 (62.5)
15
14 (93.3)/11(73.3)
S. pneumoniae 4
3 (75.0)/3 (75.0)
7
5 (71.4)/4 (57.1)
14.2 Community-Acquired Pneumonia: 7-14 day Treatment Regimen
Adult inpatients and outpatients with a diagnosis of community-acquired bacterial pneumonia were evaluated in 2 pivotal clinical studies. In the first study, 590 patients were enrolled in a prospective, multi-center, unblinded randomized trial comparing levofloxacin 500 mg once daily orally or intravenously for 7 to 14 days to ceftriaxone 1 to 2 grams intravenously once or in equally divided doses twice daily followed by cefuroxime axetil 500 mg orally twice daily for a total of 7 to 14 days. Patients assigned to treatment with the control regimen were allowed to receive erythromycin (or doxycycline if intolerant of erythromycin) if an infection due to atypical pathogens was suspected or proven. Clinical and microbiologic evaluations were performed during treatment, 5 to 7 days posttherapy, and 3 to 4 weeks posttherapy. Clinical success (cure plus improvement) with levofloxacin at 5 to 7 days posttherapy, the primary efficacy variable in this study, was superior (95%) to the control group (83%). The 95% CI for the difference of response rates (levofloxacin minus comparator) was [-6, 19]. In the second study, 264 patients were enrolled in a prospective, multi-center, non-comparative trial of 500 mg levofloxacin administered orally or intravenously once daily for 7 to 14 days. Clinical success for clinically evaluable patients was 93%. For both studies, the clinical success rate in patients with atypical pneumonia due to Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila were 96%, 96%, and 70%, respectively. Microbiologic eradication rates across both studies are presented in Table 10.
Table 10: Bacteriological Eradication Rates Across 2 Community Acquired Pneumonia Clinical Studies
Pathogen
No. Pathogens
Bacteriological Eradication Rate (%)
H. influenzae
55
98
S. pneumoniae
83
95
S. aureus
17
88
M. catarrhalis
18
94
H. parainfluenzae
19
95
K. pneumoniae
10
100.0
Community-Acquired Pneumonia Due to Multi-Drug Resistant Streptococcus pneumoniae
Levofloxacin was effective for the treatment of community-acquired pneumonia caused by multi-drug resistant Streptococcus pneumoniae (MDRSP). MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2nd generation cephalosporins (e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole). Of 40 microbiologically evaluable patients with MDRSP isolates, 38 patients (95.0%) achieved clinical and bacteriologic success at post-therapy. The clinical and bacterial success rates are shown in Table 11.
Table 11: Clinical and Bacterial Success Rates for Levofloxacin-Treated MDRSP in Community Acquired Pneumonia Patients (Population Valid for Efficacy)
Screening Susceptibility
Clinical Success
Bacteriological Success*
n/N†
%
n/N‡
%
Penicillin-resistant
16/17
94.1
16/17
94.1
2nd generation Cephalosporin resistant
31/32
96.9
31/32
96.9
Macrolide-resistant
28/29
96.6
28/29
96.6
Trimethoprim/ Sulfamethoxazole resistant
17/19
89.5
17/19
89.5
Tetracycline-resistant
12/12
100
12/12
100
* One patient had a respiratory isolate that was resistant to tetracycline, cefuroxime, macrolides and TMP/SMX and intermediate to penicillin and a blood isolate that was intermediate to penicillin and cefuroxime and resistant to the other classes. The patient is included in the database based on respiratory isolate.
† n=the number of microbiologically evaluable patients who were clinical successes; N=number of microbiologically evaluable patients in the designated resistance group.
‡ n=the number of MDRSP isolates eradicated or presumed eradicated in microbiologically evaluable patients; N=number of MDRSP isolates in a designated resistance group.
Not all isolates were resistant to all antimicrobial classes tested. Success and eradication rates are summarized in Table 12.
Table 12: Clinical Success and Bacteriologic Eradication Rates for Resistant Streptococcus pneumoniae (Community Acquired Pneumonia)
Type of Resistance
Clinical Success
Bacteriologic Eradication
Resistant to 2 antibacterials
17/18 (94.4%)
17/18 (94.4%)
Resistant to 3 antibacterials
14/15 (93.3%)
14/15 (93.3%)
Resistant to 4 antibacterials
7/7 (100%)
7/7 (100%)
Resistant to 5 antibacterials
0
0
Bacteremia with MDRSP
8/9 (89%)
8/9 (89%)
14.3 Community-Acquired Pneumonia: 5-day Treatment Regimen
To evaluate the safety and efficacy of the higher dose and shorter course of levofloxacin, 528 outpatient and hospitalized adults with clinically and radiologically determined mild to severe community-acquired pneumonia were evaluated in a double-blind, randomized, prospective, multicenter study comparing levofloxacin 750 mg, IV or orally, every day for five days or levofloxacin 500 mg IV or orally, every day for 10 days.
Clinical success rates (cure plus improvement) in the clinically evaluable population were 90.9% in the levofloxacin 750 mg group and 91.1% in the levofloxacin 500 mg group. The 95% CI for the difference of response rates (levofloxacin 750 minus levofloxacin 500) was [-5.9, 5.4]. In the clinically evaluable population (31–38 days after enrollment) pneumonia was observed in 7 out of 151 patients in the levofloxacin 750 mg group and 2 out of 147 patients in the levofloxacin 500 mg group. Given the small numbers observed, the significance of this finding cannot be determined statistically. The microbiological efficacy of the 5-day regimen was documented for infections listed in Table 13.
Table 13: Bacteriological Eradication Rates (Community-Acquired Pneumonia)
S. pneumoniae
19/20 (95%)
Haemophilus influenzae
12/12 (100%)
Haemophilus parainfluenzae
10/10 (100%)
Mycoplasma pneumoniae
26/27 (96%)
Chlamydophila pneumoniae
13/15 (87%)
14.4 Acute Bacterial Sinusitis: 5-day and 10-14 day Treatment Regimens
Levofloxacin is approved for the treatment of acute bacterial sinusitis (ABS) using either 750 mg by mouth × 5 days or 500 mg by mouth once daily × 10–14 days. To evaluate the safety and efficacy of a high dose short course of levofloxacin, 780 outpatient adults with clinically and radiologically determined acute bacterial sinusitis were evaluated in a double-blind, randomized, prospective, multicenter study comparing levofloxacin 750 mg by mouth once daily for five days to levofloxacin 500 mg by mouth once daily for 10 days.
Clinical success rates (defined as complete or partial resolution of the pre-treatment signs and symptoms of ABS to such an extent that no further antibiotic treatment was deemed necessary) in the microbiologically evaluable population were 91.4% (139/152) in the levofloxacin 750 mg group and 88.6% (132/149) in the levofloxacin 500 mg group at the test-of-cure (TOC) visit (95% CI [-4.2, 10.0] for levofloxacin 750 mg minus levofloxacin 500 mg).
Rates of clinical success by pathogen in the microbiologically evaluable population who had specimens obtained by antral tap at study entry showed comparable results for the five- and ten-day regimens at the test-of-cure visit 22 days post treatment (see Table 14).
Table 14: Clinical Success Rate by Pathogen at the TOC in Microbiologically Evaluable Subjects Who Underwent Antral Puncture (Acute Bacterial Sinusitis) Table 14: Clinical Success Rate by Pathogen at the TOC in Microbiologically Evaluable Subjects Who Underwent Antral Puncture (Acute Bacterial Sinusitis) PathogenPathogen Levofloxacin
750 mg×5 days Levofloxacin
750 mg×5 daysLevofloxacin
500 mg × 10 days Levofloxacin
500 mg × 10 daysTable 14: Clinical Success Rate by Pathogen at the TOC in Microbiologically Evaluable Subjects Who Underwent Antral Puncture (Acute Bacterial Sinusitis) Pathogen Levofloxacin
750 mg×5 daysLevofloxacin
500 mg × 10 days- *
- Note: Forty percent of the subjects in this trial had specimens obtained by sinus endoscopy. The efficacy data for subjects whose specimen was obtained endoscopically were comparable to those presented in the above table.
Streptococcus pneumoniae*
25/27 (92.6%)
26/27 (96.3%)
Haemophilus influenzae*
19/21 (90.5%)
25/27 (92.6%)
Moraxella catarrhalis*
10/11 (90.9%)
13/13 (100%)
14.5 Complicated Skin and Skin Structure Infections
Three hundred ninety-nine patients were enrolled in an open-label, randomized, comparative study for complicated skin and skin structure infections. The patients were randomized to receive either levofloxacin 750 mg once daily (IV followed by oral), or an approved comparator for a median of 10 ± 4.7 days. As is expected in complicated skin and skin structure infections, surgical procedures were performed in the levofloxacin and comparator groups. Surgery (incision and drainage or debridement) was performed on 45% of the levofloxacin-treated patients and 44% of the comparator treated patients, either shortly before or during antibiotic treatment and formed an integral part of therapy for this indication.
Among those who could be evaluated clinically 2–5 days after completion of study drug, overall success rates (improved or cured) were 116/138 (84.1%) for patients treated with levofloxacin and 106/132 (80.3%) for patients treated with the comparator.
Success rates varied with the type of diagnosis ranging from 68% in patients with infected ulcers to 90% in patients with infected wounds and abscesses. These rates were equivalent to those seen with comparator drugs.
14.6 Chronic Bacterial Prostatitis
Adult patients with a clinical diagnosis of prostatitis and microbiological culture results from urine sample collected after prostatic massage (VB3) or expressed prostatic secretion (EPS) specimens obtained via the Meares-Stamey procedure were enrolled in a multicenter, randomized, double-blind study comparing oral levofloxacin 500 mg, once daily for a total of 28 days to oral ciprofloxacin 500 mg, twice daily for a total of 28 days. The primary efficacy endpoint was microbiologic efficacy in microbiologically evaluable patients. A total of 136 and 125 microbiologically evaluable patients were enrolled in the levofloxacin and ciprofloxacin groups, respectively. The microbiologic eradication rate by patient infection at 5–18 days after completion of therapy was 75.0% in the levofloxacin group and 76.8% in the ciprofloxacin group (95% CI [-12.58, 8.98] for levofloxacin minus ciprofloxacin). The overall eradication rates for pathogens of interest are presented in Table 15.
Table 15: Bacteriological Eradication Rates (Chronic Bacterial Prostatitis)
Levofloxacin (N=136)
Ciprofloxacin (N=125)
Pathogen
N
Eradication
N
Eradication
- *
- Eradication rates shown are for patients who had a sole pathogen only; mixed cultures were excluded.
E. coli
15
14 (93.3%)
11
9 (81.8%)
E. faecalis
54
39 (72.2%)
44
33 (75.0%)
S. epidermidis*
11
9 (81.8%)
14
11 (78.6%)
Eradication rates for S. epidermidis when found with other co-pathogens are consistent with rates seen in pure isolates.
Clinical success (cure + improvement with no need for further antibiotic therapy) rates in microbiologically evaluable population 5–18 days after completion of therapy were 75.0% for levofloxacin-treated patients and 72.8% for ciprofloxacin-treated patients (95% CI [-8.87, 13.27] for levofloxacin minus ciprofloxacin). Clinical long-term success (24–45 days after completion of therapy) rates were 66.7% for the levofloxacin-treated patients and 76.9% for the ciprofloxacin-treated patients (95% CI [-23.40, 2.89] for levofloxacin minus ciprofloxacin).
14.7 Complicated Urinary Tract Infections and Acute Pyelonephritis: 5-day Treatment Regimen
To evaluate the safety and efficacy of the higher dose and shorter course of levofloxacin, 1109 patients with cUTI and AP were enrolled in a randomized, double-blind, multicenter clinical trial conducted in the US from November 2004 to April 2006 comparing levofloxacin 750 mg IV or orally once daily for 5 days (546 patients) with ciprofloxacin 400 mg IV or 500 mg orally twice daily for 10 days (563 patients). Patients with AP complicated by underlying renal diseases or conditions such as complete obstruction, surgery, transplantation, concurrent infection or congenital malformation were excluded. Efficacy was measured by bacteriologic eradication of the baseline organism(s) at the post-therapy visit in patients with a pathogen identified at baseline. The post-therapy (test-of-cure) visit occurred 10 to 14 days after the last active dose of levofloxacin and 5 to 9 days after the last dose of active ciprofloxacin.
The bacteriologic cure rates overall for levofloxacin and control at the test-of-cure (TOC) visit for the group of all patients with a documented pathogen at baseline (modified intent to treat or mITT) and the group of patients in the mITT population who closely followed the protocol (Microbiologically Evaluable) are summarized in Table 16.
Table 16: Bacteriological Eradication at Test-of-Cure
Levofloxacin
750 mg orally or IV once daily for 5 daysCiprofloxacin 400 mg IV/500 mg orally twice daily for 10 days Overall Difference [95% CI] n/N % n/N % Levofloxacin Ciprofloxacin mITT Population*
Overall (cUTI or AP)
252/333
75.7
239/318
75.2
0.5 (-6.1, 7.1)
cUTI
168/230
73.0
157/213
73.7
AP
84/103
81.6
82/105
78.1
Microbiologically Evaluable Population†
Overall (cUTI or AP)
228/265
86.0
215/241
89.2
-3.2 [-8.9, 2.5]
cUTI
154/185
83.2
144/165
87.3
AP
74/80
92.5
71/76
93.4
* The mITT population included patients who received study medication and who had a positive (≥105 CFU/mL) urine culture with no more than 2 uropathogens at baseline. Patients with missing response were counted as failures in this analysis.
†The Microbiologically Evaluable population included patients with a confirmed diagnosis of cUTI or AP, a causative organism(s) at baseline present at ≥ 105 CFU/mL, a valid test-of-cure urine culture, no pathogen isolated from blood resistant to study drug, no premature discontinuation or loss to follow-up, and compliance with treatment (among other criteria).
Microbiologic eradication rates in the Microbiologically Evaluable population at TOC for individual pathogens recovered from patients randomized to levofloxacin treatment are presented in Table 17.
Pathogen
Bacteriological Eradication Rate (n/N)
%
Escherichia coli*
155/172
90
Klebsiella pneumoniae
20/23
87
Proteus mirabilis
12/12
100
* The predominant organism isolated from patients with AP was E. coli: 91% (63/69) eradication in AP and 89% (92/103) in patients with cUTI.
14.8 Complicated Urinary Tract Infections and Acute Pyelonephritis: 10-day Treatment Regimen
To evaluate the safety and efficacy of the 250 mg dose, 10 day regimen of levofloxacin, 567 patients with uncomplicated UTI, mild-to-moderate cUTI, and mild-to-moderate AP were enrolled in a randomized, double-blind, multicenter clinical trial conducted in the US from June 1993 to January 1995 comparing levofloxacin 250 mg orally once daily for 10 days (285 patients) with ciprofloxacin 500 mg orally twice daily for 10 days (282 patients). Patients with a resistant pathogen, recurrent UTI, women over age 55 years, and with an indwelling catheter were initially excluded, prior to protocol amendment which took place after 30% of enrollment. Microbiological efficacy was measured by bacteriologic eradication of the baseline organism(s) at 1–12 days post-therapy in patients with a pathogen identified at baseline.
The bacteriologic cure rates overall for levofloxacin and control at the test-of-cure (TOC) visit for the group of all patients with a documented pathogen at baseline (modified intent to treat or mITT) and the group of patients in the mITT population who closely followed the protocol (Microbiologically Evaluable) are summarized in Table 18.
- *
- 1–9 days posttherapy for 30% of subjects enrolled prior to a protocol amendment; 5–12 days posttherapy for 70% of subjects.
- †
- The mITT population included patients who had a pathogen isolated at baseline. Patients with missing response were counted as failures in this analysis.
- ‡
- The Microbiologically Evaluable population included mITT patients who met protocol-specified evaluability criteria.
Table 18: Bacteriological Eradication Overall (cUTI or AP) at Test-Of-Cure* Levofloxacin 250 mg once daily for 10 days Ciprofloxacin 500 mg twice daily for 10 days n/N % n/N % mITT Population † 174/209 83.3 184/219 84.0 Microbiologically Evaluable Population‡ 164/177 92.7 159/171 93.0 14.9 Inhalational Anthrax (Post-Exposure)
The effectiveness of levofloxacin for this indication is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. Levofloxacin has not been tested in humans for the post-exposure prevention of inhalation anthrax. The mean plasma concentrations of levofloxacin associated with a statistically significant improvement in survival over placebo in the rhesus monkey model of inhalational anthrax are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens [see Indications and Usage (1.13) and Dosage and Administration (2.1, 2.2)].
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC0-24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively. The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily [see Clinical Pharmacology (12.3)]. Levofloxacin tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2 )].
In adults, the safety of levofloxacin for treatment durations of up to 28 days is well characterized. However, information pertaining to extended use at 500 mg daily up to 60 days is limited. Prolonged levofloxacin therapy in adults should only be used when the benefit outweighs the risk.
In pediatric patients, the safety of levofloxacin for treatment durations of more than 14 days has not been studied. An increased incidence of musculoskeletal adverse events (arthralgia, arthritis, tendinopathy, gait abnormality) compared to controls has been observed in clinical studies with treatment duration of up to 14 days. Long-term safety data, including effects on cartilage, following the administration of levofloxacin to pediatric patients is limited [see Warnings and Precautions (5.12) and Use in Specific Populations (8.4)].
A placebo-controlled animal study in rhesus monkeys exposed to an inhaled mean dose of 49 LD50 (~2.7 X 106) spores (range 17 - 118 LD50) of B. anthracis (Ames strain) was conducted. The minimal inhibitory concentration (MIC) of levofloxacin for the anthrax strain used in this study was 0.125 mcg/mL. In the animals studied, mean plasma concentrations of levofloxacin achieved at expected Tmax (1 hour post dose) following oral dosing to steady state ranged from 2.79 to 4.87 mcg/mL. Steady state trough concentrations at 24 hours post-dose ranged from 0.107 to 0.164 mcg/mL. Mean (SD) steady state AUC0-24 was 33.4 ± 3.2 mcg.h/mL (range 30.4 to 36.0 mcg.h/mL). Mortality due to anthrax for animals that received a 30 day regimen of oral levofloxacin beginning 24 hrs post exposure was significantly lower (1/10), compared to the placebo group (9/10) [P=0.0011, 2-sided Fisher's Exact Test]. The one levofloxacin treated animal that died of anthrax did so following the 30-day drug administration period.
Close14.10 Plague
Efficacy studies of levofloxacin could not be conducted in humans with pneumonic plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals.
The mean plasma concentrations of levofloxacin associated with a statistically significant improvement in survival over placebo in an African green monkey model of pneumonic plague are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens [see Indications and Usage (1.14) and Dosage and Administration (2.1), (2.2)].
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC0-24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively. The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily [see Clinical Pharmacology (12.3)]. Levofloxacin Tablets can only be administered to pediatric patients with inhalational anthrax (post-exposure) or plague who are 30 kg or greater due to the limitations of the available strengths [see Dosage and Administration (2.2) ].
A placebo-controlled animal study in African green monkeys exposed to an inhaled mean dose of 65 LD50 (range 3 to 145 LD50) of Yersinia pestis (CO92 strain) was conducted. The minimal inhibitory concentration (MIC) of levofloxacin for the Y. pestis strain used in this study was 0.03 mcg/mL. Mean plasma concentrations of levofloxacin achieved at the end of a single 30-min infusion ranged from 2.84 to 3.50 mcg/mL in African green monkeys. Trough concentrations at 24 hours post-dose ranged from <0.03 to 0.06 mcg/mL. Mean (SD) AUC0-24 was 11.9 (3.1) mcg.h/mL (range 9.50 to 16.86 mcg.h/mL). Animals were randomized to receive either a 10-day regimen of i.v. levofloxacin or placebo beginning within 6 hrs of the onset of telemetered fever (≥ 39oC for more than 1 hour). Mortality in the levofloxacin group was significantly lower (1/17) compared to the placebo group (7/7) [p<0.001, Fisher's Exact Test; exact 95% confidence interval (-99.9%, -55.5%) for the difference in mortality]. One levofloxacin-treated animal was euthanized on Day 9 post-exposure to Y. pestis due to a gastric complication; it had a blood culture positive for Y. pestis on Day 3 and all subsequent daily blood cultures from Day 4 through Day 7 were negative. -
16 HOW SUPPLIED/STORAGE AND HANDLINGLevofloxacin USP is supplied as 250 mg, 500 mg and 750 mg capsule-shaped, coated tablets. Levofloxacin tablets, USP are packaged in bottles and in unit-dose blister strips in the following ...
Levofloxacin USP is supplied as 250 mg, 500 mg and 750 mg capsule-shaped, coated tablets. Levofloxacin tablets, USP are packaged in bottles and in unit-dose blister strips in the following configurations:
- Levofloxacin tablets, USP 250 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed “T4” on one side and plain on other side.
- bottles of 50s (NDC 33342-531-08)
- bottles of 100s (NDC 33342-531-11)
- blister of 50s (NDC-33342-531-31)
- blister of 100s (NDC 33342-531-12)
- Levofloxacin tablets, USP 500 mg are peach colored, capsule shaped, film coated tablets, biconvex, debossed “T7” on one side and plain on other side.
- bottles of 50s (NDC 33342-532-08)
- bottles of 100s (NDC 33342-532-11)
- blister of 50s (NDC 33342-532-31)
- blister of 100s (NDC 33342-532-12)
- Levofloxacin tablets, USP 750 mg are white colored, capsule shaped, film coated tablets, biconvex, debossed “T5” on one side and plain on other side.
- bottles of 20s (NDC 33342-533-32)
- bottles of 100s (NDC 33342-533-11)
- blister of 50s (NDC 33342-533-31)
- blister of 100s (NDC 33342-533-12)
Levofloxacin tablets, USP should be stored at 20º to 25ºC (68º to 77°F); excursions permitted to 15º to 30ºC (59º to 86°F) in well-closed containers.
Close - Levofloxacin tablets, USP 250 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed “T4” on one side and plain on other side.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Serious Adverse Reactions - Advise patients to stop taking levofloxacin tablets if they experience an adverse ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Adverse Reactions
Advise patients to stop taking levofloxacin tablets if they experience an adverse reaction and to call their healthcare provider for advice on completing the full course of treatment with another antibacterial drug.Inform patients of the following serious adverse reactions that have been associated with levofloxacin tablets or other fluoroquinolone use:
- Disabling and Potentially Irreversible Serious Adverse Reactions That May Occur Together: Inform patients that disabling and potentially irreversible serious adverse reactions, including tendinitis and tendon rupture, peripheral neuropathies, and central nervous system effects, have been associated with use of levofloxacin tablets and may occur together in the same patient. Inform patients to stop taking levofloxacin tablets immediately if they experience an adverse reaction and to call their healthcare provider.
- Tendinitis and Tendon Rupture: Instruct patients to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue levofloxacin tablets treatment. Symptoms may be irreversible. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Peripheral Neuropathies: Inform patients that peripheral neuropathies have been associated with levofloxacin use, symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, immediately discontinue levofloxacin tablets and tell them to contact their physician.
- Central Nervous System Effects (for example, convulsions, dizziness, lightheadedness, increased intracranial pressure): Inform patients that convulsions have been reported in patients receiving fluoroquinolones, including levofloxacin. Instruct patients to notify their physician before taking this drug if they have a history of convulsions. Inform patients that they should know how they react to levofloxacin tablets before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination. Instruct patients to notify their physician if persistent headache with or without blurred vision occurs.
- Exacerbation of Myasthenia Gravis: Instruct patients to inform their physician of any history of myasthenia gravis. Instruct patients to notify their physician if they experience any symptoms of muscle weakness, including respiratory difficulties.
- Hypersensitivity Reactions: Inform patients that levofloxacin can cause hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
- Hepatotoxicity: Inform patients that severe hepatotoxicity (including acute hepatitis and fatal events) has been reported in patients taking levofloxacin tablets. Instruct patients to inform their physician if they experience any signs or symptoms of liver injury including: loss of appetite, nausea, vomiting, fever, weakness, tiredness, right upper quadrant tenderness, itching, yellowing of the skin and eyes, light colored bowel movements or dark colored urine.
- Aortic aneurysm and dissection: Inform patients to seek emergency medical care if they experience sudden chest, stomach, or back pain.
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, instruct patients to contact their physician as soon as possible.
- Prolongation of the QT Interval: Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Instruct patients to notify their physician if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- Musculoskeletal Disorders in Pediatric Patients: Instruct parents to inform their child’s physician if the child has a history of joint-related problems before taking this drug. Inform parents of pediatric patients to notify their child’s physician of any joint-related problems that occur during or following levofloxacin therapy [see Warnings and Precautions (5.12) and Use in Specific Populations (8.4)].
- Photosensitivity/Phototoxicity: Inform patients that photosensitivity/phototoxicity has been reported in patients receiving fluoroquinolones. Inform patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking fluoroquinolones. If patients need to be outdoors while using fluoroquinolones, instruct them to wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, instruct patients to contact their physician.
- Lactation: Advise a lactating woman that she may pump and discard during treatment with levofloxacin and for an additional 2 days after the last dose. Alternatively, advise a lactating woman that breastfeeding is not recommended during treatment with levofloxacin and for an additional 2 days after the last dose [see Use in Specific Populations (8.2)].
Antibacterial Resistance
Antibacterial drugs including levofloxacin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When levofloxacin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by levofloxacin or other antibacterial drugs in the future.
Administration with Food, Fluids, and Concomitant MedicationsPatients should be informed that levofloxacin tablets may be taken with or without food. The tablets should be taken at the same time each day.
Patients should drink fluids liberally while taking levofloxacin tabletsto avoid formation of a highly concentrated urine and crystal formation in the urine.
Antacids containing magnesium, or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine should be taken at least two hours before or two hours after oral levofloxacin administration
Drug Interactions with Insulin, Oral Hypoglycemic Agents, and Warfarin
Patients should be informed that if they are diabetic and are being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, they should discontinue levofloxacin tablets and consult a physician.
Patients should be informed that concurrent administration of warfarin and levofloxacin tablets has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin, be monitored for evidence of bleeding, and also have their anticoagulation tests closely monitored while taking warfarin concomitantly.
Plague and Anthrax Studies
Patients given levofloxacin tablets for these conditions should be informed that efficacy studies could not be conducted in humans for ethical and feasibility reasons. Therefore, approval for these conditions was based on efficacy studies conducted in animals.
Manufactured for:
Macleods Pharma USA, Inc.,
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceutical Ltd.
Baddi, Himachal Pradesh, India.
Medication Guide available at:www.macleodspharma.com/usa
Revised :01/2023
Close -
MEDICATION GUIDEMedication Guide - Levofloxacin Tablets - (LEE-voe-FLOX-a-sin) What is the most important information I should know about levofloxacin tablets? Levofloxacin tablets, a fluoroquinolone ...
Medication Guide
Levofloxacin Tablets
(LEE-voe-FLOX-a-sin)What is the most important information I should know about levofloxacin tablets?
Levofloxacin tablets, a fluoroquinolone antibiotic, can cause serious side effects. Some of these serious side effects can happen at the same time and could result in death.
If you have any of the following serious side effects while you take levofloxacin tablets, you should stop taking levofloxacin tablets immediately and get medical help right away.
1. Tendon rupture or swelling of the tendon (tendinitis).- Tendon problems can happen in people of all ages who take levofloxacin tablets. Tendons are tough cords of tissue that connect muscles to bones.
Some tendon problems include:
-
- pain
- swelling
- tears, and swelling of tendons including the back of the ankle (Achilles), shoulder, hand, or other tendon sites.
- The risk of getting tendon problems while you take levofloxacin tablets is higher if you:
- are over 60 years of age
- are taking steroids (corticosteroids)
- have had a kidney, heart or lung transplant.
- Tendon problems can happen in people who do not have the above risk factors when they take levofloxacin tablets.
- Other reasons that can increase your risk of tendon problems can include:
- physical activity or exercise
- kidney failure
- tendon problems in the past, such as in people with rheumatoid arthritis (RA)
- Stop taking levofloxacin tablets immediately and get medical help right away at the first sign of tendon pain, swelling or inflammation. Avoid exercise and using the affected area.
- The most common area of pain and swelling is the Achilles tendon at the back of your ankle. This can also happen with other tendons. You may need a different antibiotic that is not a fluoroquinolone to treat your infection.
- Tendon rupture can happen while you are taking or after you have finished taking levofloxacin tablets. Tendon ruptures can happen within hours or days of taking levofloxacin tablets and have happened up to several months after people have finished taking their fluoroquinolone.
- Stop taking levofloxacin tablets immediately and get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
- hear or feel a snap or pop in a tendon area
- bruising right after an injury in a tendon area
- unable to move the affected area or bear weight
The tendon problems may be permanent.
2. Changes in sensation and possible nerve damage (Peripheral Neuropathy). Damage to the nerves in arms, hands, legs, or feet can happen in people who take fluoroquinolones, including levofloxacin tablets. Stop taking levofloxacin tablets immediately and talk to your healthcare provider right away if you get any of the following symptoms of peripheral neuropathy in your arms, hands, legs, or feet:
• pain • numbness
• burning • weakness
• tinglingThe nerve damage may be permanent
3. Central Nervous System (CNS) effects. Mental health problems and seizures have been reported in people who take fluoroquinolone antibacterial medicines, including levofloxacin tablets. Tell your healthcare provider if you have a history of mental health problems, including depression, or have a history of seizures before you start taking levofloxacin tablets. CNS side effects may happen as soon as after taking the first dose of levofloxacin tablets. Stop taking levofloxacin tablets immediately and talk to your healthcare provider right away if you get any of these side effects, or other changes in mood or behavior:
• seizures • nightmares
• hear voices, see things, or sense things that are not there(hallucinations) • feel lightheaded or dizzy
• feel restless or agitated • feel more suspicious (paranoia)
• tremors • suicidal thoughts or acts
• feel anxious or nervous • headaches that will not go away, with or without blurred vision
• confusion • memory problems
• depression • false or strange thoughts or beliefs (delusion)
• reduced awareness of surroundings
• trouble sleeping
The CNS changes may be permanent.
4. Worsening of myasthenia gravis (a problem that causes muscle weakness). Fluoroquinolones like levofloxacin tablets may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Tell your healthcare provider if you have a history of myasthenia gravis before you start taking levofloxacin tablets. Call your healthcare provider right away if you have any worsening muscle weakness or breathing problems.
What are levofloxacin tablets?
Levofloxacin tablets are a fluoroquinolone antibiotic medicine used in adults age 18 years or older to treat certain infections caused by certain germs called bacteria. These bacterial infections include:• nosocomial pneumonia • plague
• community acquired pneumonia • urinary tract infections, complicated and uncomplicated• skin infections, complicated and uncomplicated • acute kidney infection (pyelonephritis)
• chronic prostate infection • acute sinus infection
• inhalation anthrax germs • acute worsening or chronic bronchitis
Studies of levofloxacin tablets for use in the treatment of plague and anthrax were done in animals only, because plague and anthrax could not be studied in people.
Levofloxacin tablets should not be used in people with uncomplicated urinary tract infections, acute bacterial exacerbation of chronic bronchitis, or acute bacterial sinusitis if there are other treatment options available.
Levofloxacin is also used to treat children who weigh at least 66 pounds (or at least 30 kilograms) and may have breathed in anthrax germs, have plague, or been exposed to plague germs.
It is not known if levofloxacin tablets are safe and effective in children under 6 months of age.
The safety and effectiveness in children treated with levofloxacin tablets for more than 14 days is not known.
Who should not take levofloxacin tablets?
Do not take levofloxacin tablets if you have ever had a severe allergic reaction to an antibiotic known as a fluoroquinolone, or if you are allergic to levofloxacin or any of the ingredients in levofloxacin tablets. See the end of this leaflet for a complete list of ingredients in levofloxacin tablets.
Before you take levofloxacin tablets, tell your healthcare provider about all of your medical conditions, including if you:- have tendon problems. Levofloxacin tablets should not be used in people who have a history of tendon problems.
- have a problem that causes muscle weakness (myasthenia gravis). Levofloxacin tablets should not be used in people who have a known history of myasthenia gravis.
- have a history of mental health problems, including depression.
- have central nervous system problems such as seizures (epilepsy).
- have nerve problems. Levofloxacin should not be used in people who have a history of a nerve problem called peripheral neuropathy.
- have or anyone in your family has an irregular heartbeat, especially a condition called “QT prolongation”.
- have low blood potassium (hypokalemia).
- have bone problems.
- have joint problems including rheumatoid arthritis (RA).
- have kidney problems. You may need a lower dose of levofloxacin tablets if your kidneys do not work well.
- have liver problems.
- have diabetes or problems with low blood sugar (hypoglycemia).
- are pregnant or plan to become pregnant. Levofloxacin tablets will harm your unborn child.
- are breastfeeding or plan to breastfeed. Levofloxacin passes into your breast milk. You should not breastfeed during treatment with levofloxacin and for 2 days after taking your last dose of levofloxacin. You may pump your breast milk and throw it away during treatment with levofloxacin and for 2 days after taking your last dose of levofloxacin. If you are taking levofloxacin for inhalational anthrax, you and your healthcare provider should decide whether you can continue breastfeeding while taking levofloxacin.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Levofloxacin tablets and other medicines can affect each other causing side effects.
Especially tell your healthcare provider if you take:
• a steroid medicine.
• an anti-psychotic medicine
• a tricyclic antidepressant
• a water pill (diuretic)
• certain medicines may keep levofloxacin tablets from working correctly. Take levofloxacin tablets either 2 hours before or 2 hours after taking these medicines or supplements:
o an antacid, multivitamin, or other medicines or supplements that have magnesium, aluminum, iron, or zinc
o sucralfate (Carafate®)
o didanosine (Videx®,Videx® EC)• a blood thinner (warfarin, Coumadin, Jantoven).
• an oral anti-diabetes medicine or insulin.
• an NSAID (Non-Steroidal Anti-Inflammatory Drug).
Many common medicines for pain relief are NSAIDs. Taking an NSAID while you take levofloxacin tablets or other fluoroquinolones may increase your risk of central nervous system effects and seizures.
• theophylline (Theo-24®, Elixophyllin®, Theochron®, Uniphyl®, Theolair®).
• a medicine to control your heart rate or rhythm (antiarrhythmics).Ask your healthcare provider if you are not sure if any of your medicines are listed above.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.How should I take levofloxacin tablets?
• Take levofloxacin exactly as your healthcare provider tells you to take it.
• Take levofloxacin at the same time each day.• Drink plenty of fluids while you take levofloxacin.
• Levofloxacin can be taken with or without food.
If you miss a dose of levofloxacin tablets and it is:
o 8 hours or more until your next scheduled dose, take your missed dose right away. Then take the next dose at your regular time.
o less than 8 hours until your next scheduled dose, do not take the missed dose. Take the next dose at your regular time.• Do not skip any doses of levofloxacin or stop taking it, even if you begin to feel better, until you finish your prescribed treatment unless:
o you have tendon problems. See “What is the most important information I should know about levofloxacin ?”.o you have a nerve problem. See “What is the most important information I should know about levofloxacin ?”.
o you have a central nervous system problem. See “What is the most important information I should know about levofloxacin ?”.
o you have a serious allergic reaction. See “What are the possible side effects of levofloxacin ?”.
o your healthcare provider tells you to stop taking levofloxacin
Taking all of your levofloxacin tablets doses will help make sure that all of the bacteria are killed. Taking all of your levofloxacin tablets doses will help you lower the chance that the bacteria will become resistant to levofloxacin tablets. If your infection does not get better while you take levofloxacin tablets, it may mean that the bacteria causing your infection may be resistant to levofloxacin tablets. If your infection does not get better, call your healthcare provider. If your infection does not get better, levofloxacin tablets and other similar antibiotic medicines may not work for you in the future.
• If you take too much levofloxacin tablets, call your healthcare provider or get medical help right away.
What should I avoid while taking levofloxacin tablets?
• Levofloxacin tablets can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how levofloxacin tablets affects you.
• Avoid sunlamps, tanning beds, and try to limit your time in the sun. Levofloxacin tablets can make your skin sensitive to the sun (photosensitivity) and the light from sunlamps and tanning beds. You could get severe sunburn, blisters or swelling of your skin. If you get any of these symptoms while you take levofloxacin tablets, call your healthcare provider right away. You should use sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight.What are the possible side effects of levofloxacin tablets?
Levofloxacin tablets may cause serious side effects, including:
• See “What is the most important information I should know about levofloxacin tablets?”• Serious allergic reactions. Allergic reactions can happen in people taking fluoroquinolones, including levofloxacin tablets, even after only 1 dose. Stop taking levofloxacin tablets and get emergency medical help right away if you have any of the following symptoms of a severe allergic reaction:
o hives o rapid heartbeat
o trouble breathing or swallowing o faint
o swelling of the lips, tongue, face o skin rash
o throat tightness, hoarseness
Skin rash may happen in people taking levofloxacin tablets, even after only 1 dose. Stop taking levofloxacin tablets at the first sign of a skin rash and immediately call your healthcare provider. Skin rash may be a sign of a more serious reaction to levofloxacin tablets.
• Liver damage (hepatotoxicity): Hepatotoxicity can happen in people who take levofloxacin tablets. Call your healthcare provider right away if you have unexplained symptoms such as:
o nausea or vomiting o unusual tiredness
o stomach pain o loss of appetite
o fever o light colored bowel movements
o weakness o dark colored urine
o pain or tenderness in the upper right side of your stomach-area o yellowing of your skin or the whites of your eyes
o itching
Stop taking levofloxacin tablets and tell your healthcare provider right away if you have yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to levofloxacin tablets (a liver problem).
• Aortic aneurysm and dissection
Tell your healthcare provider if you have ever been told that you have an aortic aneurysm, a swelling of the large artery that carries blood from the heart to the body. Get emergency medical help right away if you have sudden chest, stomach, or back pain.• Intestine infection (Clostridium difficile-associated diarrhea). Clostridium difficile-associated diarrhea (CDAD) can happen with many antibiotics, including levofloxacin. Call your healthcare provider right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. CDAD can happen 2 or more months after you have finished your antibiotic.
• Serious heart rhythm changes (QT prolongation and torsades de pointes)
Tell your healthcare provider right away if you have a change in your heart beat (a fast or irregular heartbeat), or if you faint. Levofloxacin tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:
o who are elderly
o with a family history of prolonged QT interval
o with low blood potassium (hypokalemia)
o who take certain medicines to control heart rhythm (antiarrhythmics)• Joint Problems
Increased chance of problems with joints and tissues around joints in children can happen. Tell your child’s healthcare provider if your child has any joint problems during or after treatment with levofloxacin tablets.
• Changes in blood sugar
People who take levofloxacin tablets and other fluoroquinolone medicines with oral anti-diabetes medicines or with insulin can get low blood sugar (hypoglycemia) and high blood sugar (hyperglycemia). Follow your healthcare provider’s instructions for how often to check your blood sugar. If you have diabetes and you get low blood sugar while taking levofloxacin tablets, stop taking levofloxacin tablets and call your healthcare provider right away. Your antibiotic medicine may need to be changed.• Sensitivity to sunlight (photosensitivity)
See “What should I avoid while taking levofloxacin tablets?”
The most common side effects of levofloxacin tablets include:
o nausea o insomnia
o headache o constipation
o diarrhea o dizzinessIn children 6 months and older who take levofloxacin to treat anthrax disease or plague, vomiting is also common.
Levofloxacin tablets may cause false-positive urine screening results for opiates when testing is done with some commercially available kits. A positive result should be confirmed using a more specific test.
These are not all the possible side effects of levofloxacin tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
How should I store levofloxacin tablets?• Store levofloxacin tablets at room temperature between 59°F to 86° F (15°C to 30°C).
• Keep levofloxacin tablets in a tightly closed container.General information about the safe and effective use of levofloxacin tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use levofloxacin tablets for a condition for which it is not prescribed. Do not give levofloxacin tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about levofloxacin tablets. If you would like more information about levofloxacin tablets, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about levofloxacin tablets that is written for health professionals.
For more information, call 1-888-943-3210 or 1-855-926-3384
What are the ingredients in levofloxacin tablets?Active ingredient: levofloxacin.
All Levofloxacin tablets:Inactive ingredients: hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80.
Levofloxacin tablets 250 mg also contain synthetic red iron oxide, levofloxacin tablets 500 mg also contain synthetic red and yellow iron oxides.
The brands listed are trademarks of their respective owners.
Manufactured for:
Macleods Pharma USA, Inc.,
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceutical Ltd.
Baddi, Himachal Pradesh, India.This Medication Guide has been approved by the U.S. Food and Drug Administration.
Medication Guide available at:www.macleodspharma.com/usa
Revised: January 2023
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELLevofloxacin Tablets, USP - 250 mg – Bottle of 50s - NDC 33342-531-08 - Levofloxacin Tablets, USP - 250 mg – Bottle of 100s - NDC 33342-531-11 - Levofloxacin Tablets, USP - 500 mg – Bottle of 50s ...
Levofloxacin Tablets, USP
250 mg – Bottle of 50s
NDC 33342-531-08
Levofloxacin Tablets, USP
250 mg – Bottle of 100s
NDC 33342-531-11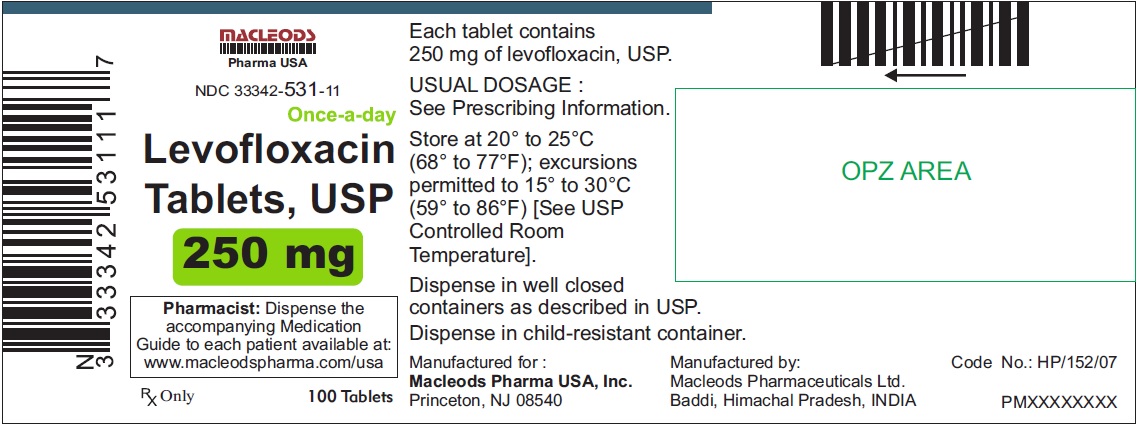
Levofloxacin Tablets, USP
500 mg – Bottle of 50s
NDC 33342-532-08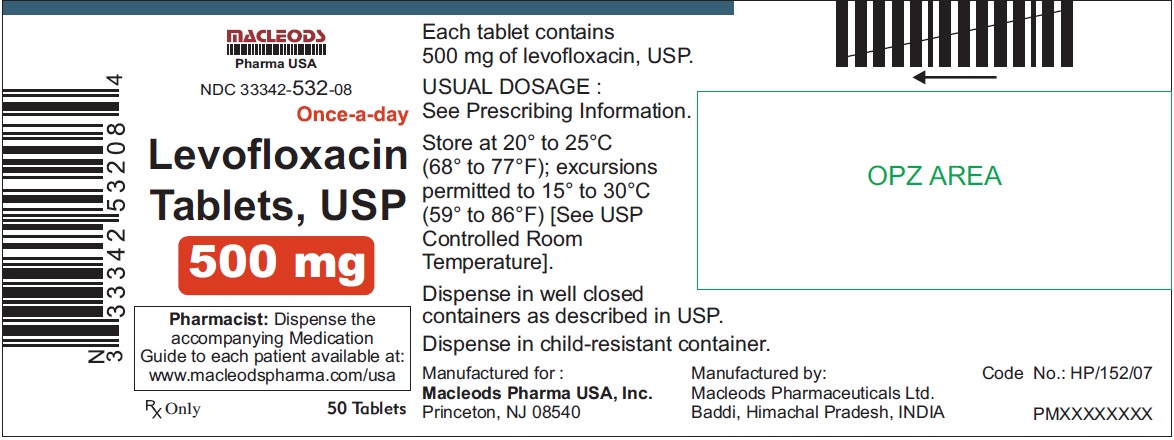
Levofloxacin Tablets, USP
500 mg – Bottle of 100s
NDC 33342-532-11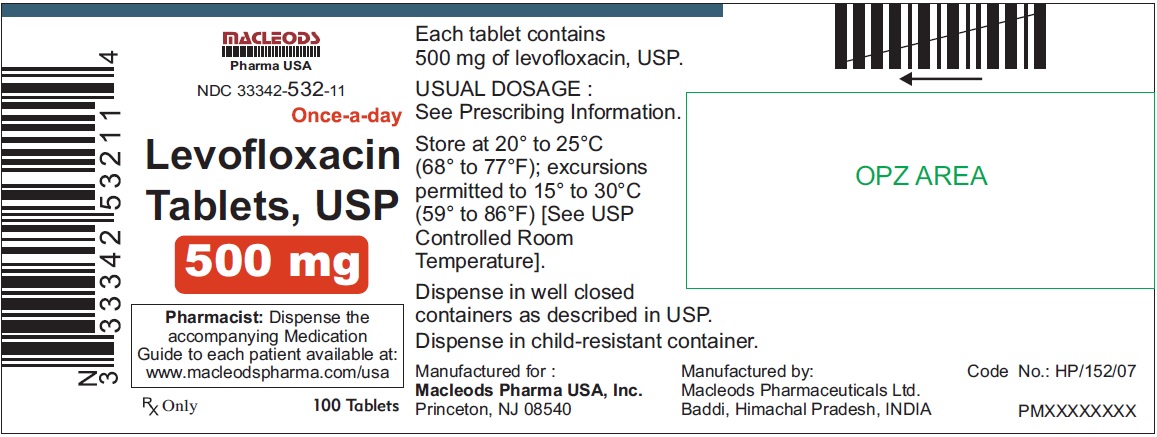
Levofloxacin Tablets, USP
750 mg – Bottle of 20 Tablets
NDC 33342-533-32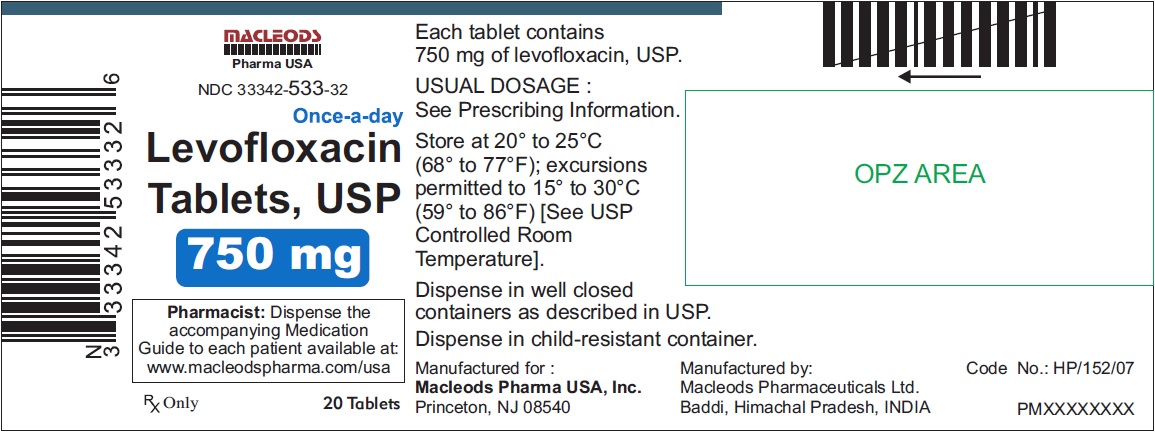
Levofloxacin Tablets, USP
750 mg – Bottle of 100 Tablets
NDC 33342-533-11
Close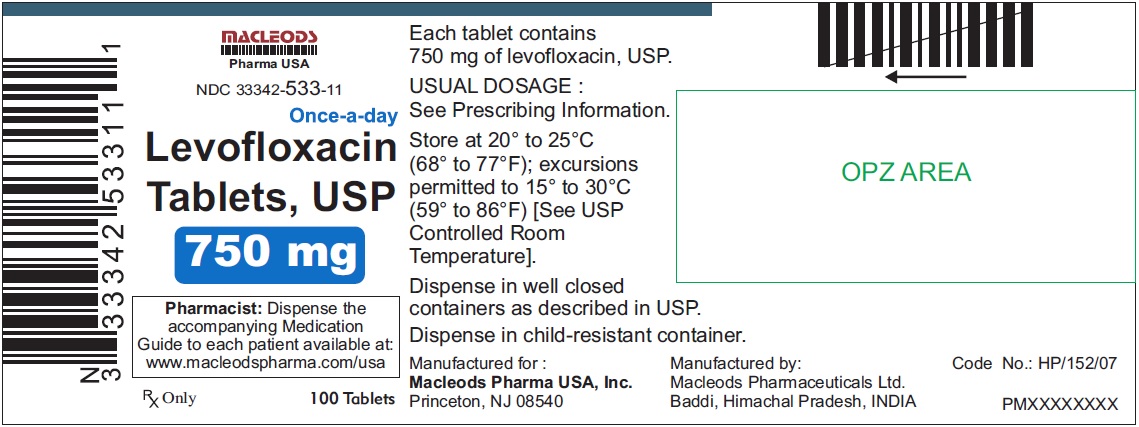
-
INGREDIENTS AND APPEARANCEProduct Information
LEVOFLOXACIN levofloxacin tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-531 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOFLOXACIN (UNII: 6GNT3Y5LMF) (LEVOFLOXACIN ANHYDROUS - UNII:RIX4E89Y14) LEVOFLOXACIN ANHYDROUS 250 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color PINK (pink) Score no score Shape CAPSULE (capsule) Size 15mm Flavor Imprint Code T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-531-08 50 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 2 NDC:33342-531-31 50 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 3 NDC:33342-531-12 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 4 NDC:33342-531-11 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200839 03/22/2012 LEVOFLOXACIN levofloxacin tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-532 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOFLOXACIN (UNII: 6GNT3Y5LMF) (LEVOFLOXACIN ANHYDROUS - UNII:RIX4E89Y14) LEVOFLOXACIN ANHYDROUS 500 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color ORANGE Score no score Shape CAPSULE (capsule) Size 19mm Flavor Imprint Code T7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-532-08 50 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 2 NDC:33342-532-31 50 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 3 NDC:33342-532-12 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 4 NDC:33342-532-11 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200839 03/22/2012 LEVOFLOXACIN levofloxacin tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-533 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOFLOXACIN (UNII: 6GNT3Y5LMF) (LEVOFLOXACIN ANHYDROUS - UNII:RIX4E89Y14) LEVOFLOXACIN ANHYDROUS 750 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE (white) Score no score Shape CAPSULE (capsule) Size 22mm Flavor Imprint Code T5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-533-32 20 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 2 NDC:33342-533-31 50 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 3 NDC:33342-533-12 100 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/22/2012 4 NDC:33342-533-11 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200839 03/22/2012 Labeler - Macleods Pharmaceuticals Limited (862128535)
CloseEstablishment Name Address ID/FEI Business Operations Macleods Pharmaceuticals Limited 676369519 ANALYSIS(33342-531, 33342-532, 33342-533) , LABEL(33342-531, 33342-532, 33342-533) , MANUFACTURE(33342-531, 33342-532, 33342-533) , PACK(33342-531, 33342-532, 33342-533)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LEVOFLOXACIN tablet, film coated
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jan 10, 2023 | 1 (current) | download |
RxNorm
LEVOFLOXACIN tablet, film coated
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 199884 | levoFLOXacin 250 MG Oral Tablet | PSN |
| 2 | 199884 | levofloxacin 250 MG Oral Tablet | SCD |
| 3 | 199884 | levofloxacin (as levofloxacin hemihydrate) 250 MG Oral Tablet | SY |
| 4 | 199885 | levoFLOXacin 500 MG Oral Tablet | PSN |
| 5 | 199885 | levofloxacin 500 MG Oral Tablet | SCD |
| 6 | 311296 | levoFLOXacin 750 MG Oral Tablet | PSN |
| 7 | 311296 | levofloxacin 750 MG Oral Tablet | SCD |
Get Label RSS Feed for this Drug
LEVOFLOXACIN tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=fc165a6d-24b0-4753-a7ac-94a134dca0d3
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LEVOFLOXACIN tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 33342-531-08 |
| 2 | 33342-531-11 |
| 3 | 33342-531-12 |
| 4 | 33342-531-31 |
| 5 | 33342-532-08 |
| 6 | 33342-532-11 |
| 7 | 33342-532-12 |
| 8 | 33342-532-31 |
| 9 | 33342-533-11 |
| 10 | 33342-533-12 |
| 11 | 33342-533-31 |
| 12 | 33342-533-32 |