Label: FEBUXOSTAT- febuxostat tablets 40 mg film
FEBUXOSTAT- febuxostat tablets 80 mg film
- NDC Code(s): 33342-274-07, 33342-274-10, 33342-274-15, 33342-275-07, view more
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FEBUXOSTAT TABLETS safely and effectively. See full prescribing information for FEBUXOSTAT TABLETS. FEBUXOSTAT tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING : CARDIOVASCULAR DEATH
Close
Gout patients with established cardiovascular (CV) disease treated with febuxostat had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study [see Warnings and Precautions (5.1)].
Consider the risks and benefits of febuxostat when deciding to prescribe or continue patients on febuxostat. Febuxostat should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable [see Indications and Usage (1)]. -
1 INDICATIONS & USAGEFebuxostat is a xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of ...
-
2 DOSAGE & ADMINISTRATION2.1 Recommended Dosage - The recommended febuxostat tablets dosage is 40 mg or 80 mg once daily. The recommended starting dosage of febuxostat tablets is 40 mg once daily. For patients who do ...
-
3 DOSAGE FORMS & STRENGTHS40 mg tablets, green, biconvex, round, film coated tablets debossed with "M 87" on one side and plain on the other side. 80 mg tablets, green, biconvex, teardrop shaped, film coated tablets ...
-
4 CONTRAINDICATIONSFebuxostat is contraindicated in patients being treated with azathioprine or mercaptopurine [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Death - In a cardiovascular (CV) outcome study, gout patients with established CV disease treated with febuxostat had a higher rate of CV death compared to those treated with ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the prescribing information: • Cardiovascular Death [see Warnings and Precautions (5.1)] • Hepatic Effects [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Xanthine Oxidase Substrate Drugs - Febuxostat is an XO inhibitor. A drug interaction study of febuxostat and azathioprine, also metabolized by XO, showed an increase in exposure of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with febuxostat use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. No adverse ...
-
10 OVERDOSAGEFebuxostat was studied in healthy patients in doses up to 300 mg daily for seven days without evidence of dose-limiting toxicities. No overdose of febuxostat was reported in clinical studies ...
-
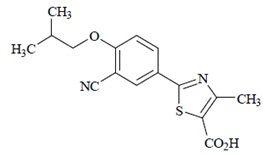
11 DESCRIPTIONFebuxostat is a xanthine oxidase inhibitor. The active ingredient in febuxostat is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Febuxostat, a xanthine oxidase inhibitor, achieves its therapeutic effect by decreasing serum uric acid. Febuxostat is not expected to inhibit other enzymes involved in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility - Two year carcinogenicity studies were conducted in F344 rats and B6C3F1 mice. Increased transitional cell papilloma and carcinoma of ...
-
14 CLINICAL STUDIESA serum uric acid level of less than 6 mg/dL is the goal of antihyperuricemic therapy and has been established as appropriate for the treatment of gout. 14.1 Management of Hyperuricemia in ...
-
PATIENT PACKAGE INSERT.
-
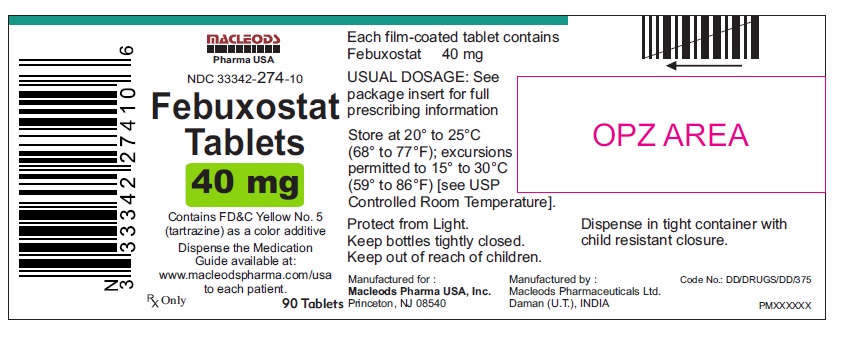
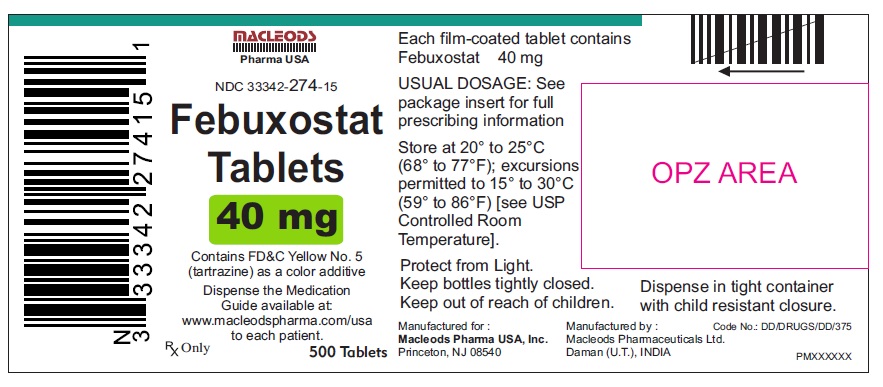
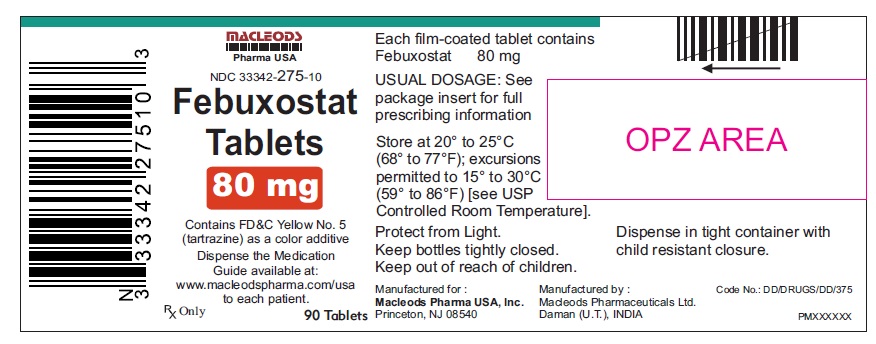
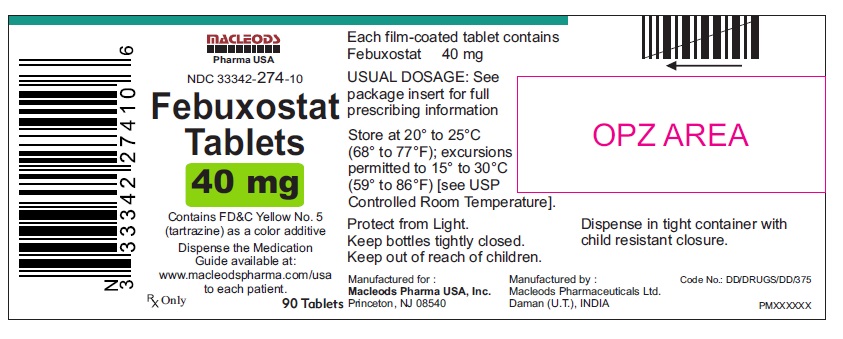
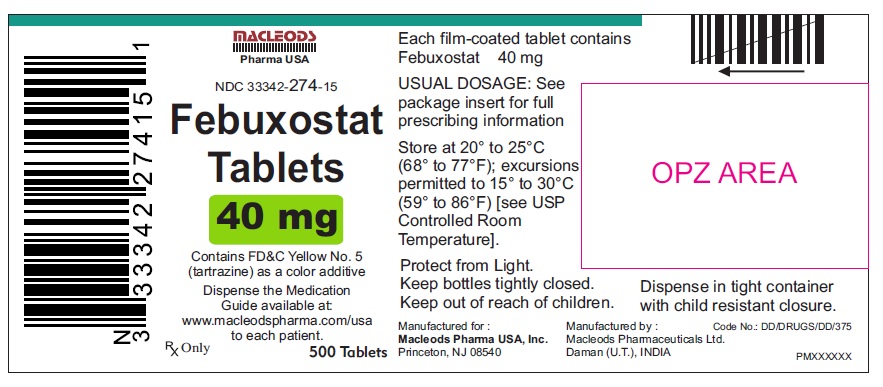
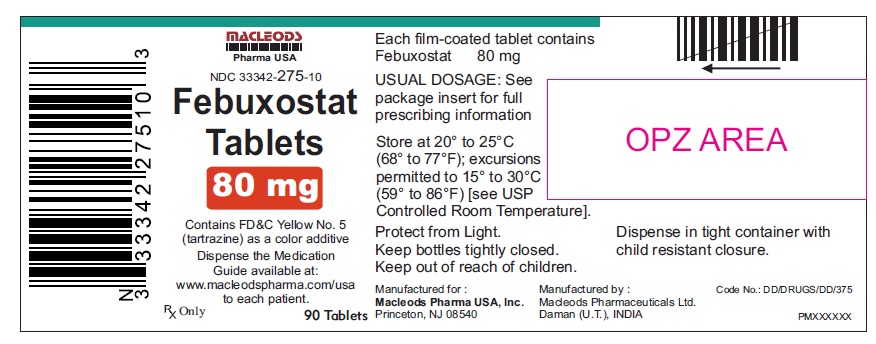
16 HOW SUPPLIED/STORAGE AND HANDLINGFebuxostat 40 mg tablets are green, biconvex, round, film- coated tablets debossed with "M 87" on one side and plain on the other side and supplied as: NDC Number Size ...
-
17 PATIENT COUNSELING INFORMATIONCV Death - Inform patients that gout patients with established CV disease treated with febuxostat had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study ...
-
MEDICATION GUIDEDispense with Medication Guide available at: www.macleodspharma.com/usa - Febuxostat(feb ux' oh stat) (febuxostat) tablets, for oral use - Read the Medication Guide that comes with Febuxostat ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFebuxostat Tablets 40mg - Bottles of 30's - NDC Code: 33342-274-07 - Febuxostat Tablets 40mg - Bottles of 90's - NDC Code: 33342-274-10 - Febuxostat Tablets 40mg - Bottles of 500's ...
-
INGREDIENTS AND APPEARANCEProduct Information