Label: LEVOCETIRIZINE DIHYDROCHLORIDE tablet, film coated
- NDC Code(s): 33342-200-07, 33342-200-10
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOCETIRIZINE DIHYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for LEVOCETIRIZINE DIHYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.2 Chronic Idiopathic Urticaria - Levocetirizine dihydrochloride tablets are indicated for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in adults and ...
-
2 DOSAGE & ADMINISTRATIONLevocetirizine dihydrochloride is available as 5 mg breakable (scored) tablets, allowing for the administration of 2.5 mg, if needed. Levocetirizine dihydrochloride can be taken without regard to ...
-
3 DOSAGE FORMS & STRENGTHSLevocetirizine dihydrochloride tablets, USP are white to off-white, film-coated, biconvex, oval-shaped, scored on both sides and imprinted with ‘M 17’ on one side, and contain 5 mg levocetirizine ...
-
4 CONTRAINDICATIONSThe use of levocetirizine dihydrochloride is contraindicated in: 4.1 Patients with Known Hypersensitivity - Patients with known hypersensitivity to levocetirizine or any of the ingredients of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Somnolence - In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with levocetirizine dihydrochloride. Patients should be ...
-
6 ADVERSE REACTIONSUse of levocetirizine dihydrochloride has been associated with somnolence, fatigue, asthenia, and urinary retention [see Warnings and Precautions (5)]. 6.1 Clinical Trials Experience - The safety ...
-
7 DRUG INTERACTIONSIn vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience with levocetirizine use in pregnant women are insufficient to identify any drug-associated ...
-
10 OVERDOSAGEOverdosage has been reported with levocetirizine dihydrochloride. Symptoms of overdose may include drowsiness in adults. In children agitation and restlessness may initially occur, followed by ...
-
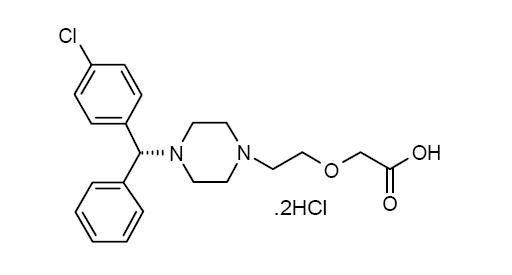
11 DESCRIPTIONLevocetirizine dihydrochloride, USP the active component of levocetirizine dihydrochloride tablets, USP is an orally active H1-receptor antagonist. The chemical name is (R)-[2-[4-[(4-chlorophenyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Levocetirizine, the active enantiomer of cetirizine, is an antihistamine; its principal effects are mediated via selective inhibition of H1 receptors. The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility - No carcinogenicity studies have been performed with levocetirizine. However, evaluation of cetirizine carcinogenicity studies is ...
-
14 CLINICAL STUDIES14.1 Perennial Allergic Rhinitis - Adults and Adolescents 12 Years of Age and Older - The efficacy of levocetirizine dihydrochloride tablet was evaluated in four randomized, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLevocetirizine dihydrochloride tablets, USP are white to off white, film-coated, biconvex, oval-shaped, scored, debossed with 'M 17' on one side and contain 5 mg levocetirizine dihydrochloride ...
-
17 PATIENT COUNSELING INFORMATIONSomnolence - Caution patients against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELRx Only - NDC 33342-200-07 - Levocetirizine Dihydrochloride tablets, USP 5 mg - Bottle of 30 tablets - Rx Only - NDC 33342-200-10 - Levocetirizine Dihydrochloride tablets, USP 5 mg - Bottle of 90 ...
-
INGREDIENTS AND APPEARANCEProduct Information