Label: VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet
-
NDC Code(s):
33342-074-10,
33342-074-44,
33342-075-10,
33342-075-15, view more33342-076-10, 33342-076-15, 33342-077-10, 33342-077-15, 33342-078-10, 33342-078-15
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALSARTAN and HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for VALSARTAN and ...These highlights do not include all the information needed to use VALSARTAN and HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for VALSARTAN and HYDROCHLOROTHIAZIDE TABLETS.
VALSARTAN and HYDROCHLOROTHIAZIDE tablets, for oral use
Initial U.S. Approval: 1998
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue valsartan and hydrochlorothiazide tablets as soon as possible. (5.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1)
INDICATIONS AND USAGE
Valsartan and hydrochlorothiazide tablets are the combination tablet of valsartan, an angiotensin II receptor blocker (ARB) and hydrochlorothiazide (HCTZ), a diuretic.
Valsartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure :
• In patients not adequately controlled with monotherapy (1)
• As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals (1)
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets (valsartan/HCTZ mg): 80/12.5, 160/12.5, 160/25, 320/12.5, 320/25 (3)
CONTRAINDICATIONS
Anuria; Hypersensitivity to any sulfonamide-derived drugs or any component; Do not coadminister aliskiren with valsartan and hydrochlorothiazide tablets in patients with diabetes (4)
WARNINGS AND PRECAUTIONS
• Hypotension: Correct volume depletion prior to initiation (5.2)
• Observe for signs of fluid or electrolyte imbalance (5.9)
• Monitor renal function and potassium in susceptible patients (5.3,5.7)
• Exacerbation or activation of systemic lupus erythematosus (5.5)
• Acute angle-closure glaucoma (5.8)ADVERSE REACTIONS
The most common reasons for discontinuation of therapy with valsartan and hydrochlorothiazide were headache and dizziness. The only adverse experience that occurred in ≥2% of patients treated with valsartan and hydrochlorothiazide and at a higher incidence than placebo was nasopharyngitis (2.4% vs. 1.9%).(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA, Inc., at 1-888-943-3210 or 1-855-926-3384 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required (7)
- Cholestyramine and colestipol: Reduced absorption of thiazides (12.3)
- Lithium: Increased risk of lithium toxicity. Monitor serum lithium concentrations during concurrent use. (7)
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): May increase risk of renal impairment. Can reduce diuretic, natriuretic and antihypertensive effects of diuretics. (7)
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia (7)
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding is not recommended (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING:FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
2.2 Add-On Therapy
2.3 Replacement Therapy
2.4 Initial Therapy
2.5 Use with Other Antihypertensive Drugs
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Hypotension in Volume- and/or Salt-Depleted Patients
5.3 Impaired Renal Function
5.4 Hypersensitivity Reaction
5.5 Systemic Lupus Erythematosus
5.6 Lithium Interaction
5.7 Potassium Abnormalities
5.8 Acute Myopia and Secondary Angle-Closure Glaucoma
5.9 Metabolic Disturbances
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypertension
14.2 Initial Therapy - Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGEValsartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal ...
Valsartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including hydrochlorothiazide and the angiotensin II receptor blocker (ARB) class to which valsartan principally belongs. There are no controlled trials demonstrating risk reduction with valsartan and hydrochlorothiazide tablets.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality have also been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (e.g., patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Add-On Therapy
Valsartan and hydrochlorothiazide tablets may be used in patients whose blood pressure is not adequately controlled on monotherapy.
Replacement Therapy
Valsartan and hydrochlorothiazide tablets may be substituted for the titrated components.
Initial Therapy
Valsartan and hydrochlorothiazide tablets may be used as initial therapy in patients who are likely to need multiple drugs to achieve blood pressure goals.
The choice of valsartan and hydrochlorothiazide tablets as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 hypertension are at a relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient's risk.
Data from the high dose multifactorial trial [see Clinical Studies (14.1)]provides estimates of the probability of reaching a target blood pressure with valsartan and hydrochlorothiazide tablets compared to valsartan or hydrochlorothiazide monotherapy. The figures below provide estimates of the likelihood of achieving systolic or diastolic blood pressure control with valsartan and hydrochlorothiazide tablets 320/25 mg, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
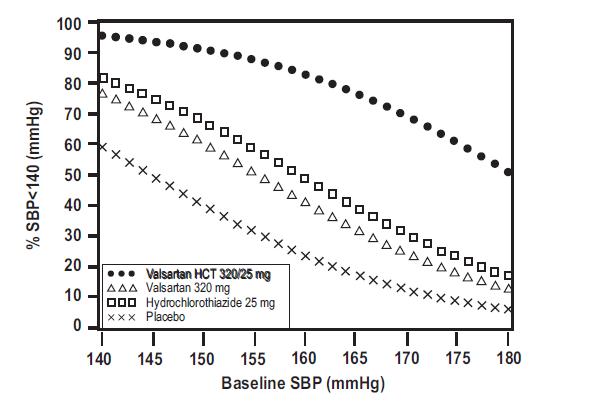
Figure 1: Probability of Achieving Systolic Blood Pressure <140 mmHg at Week 8
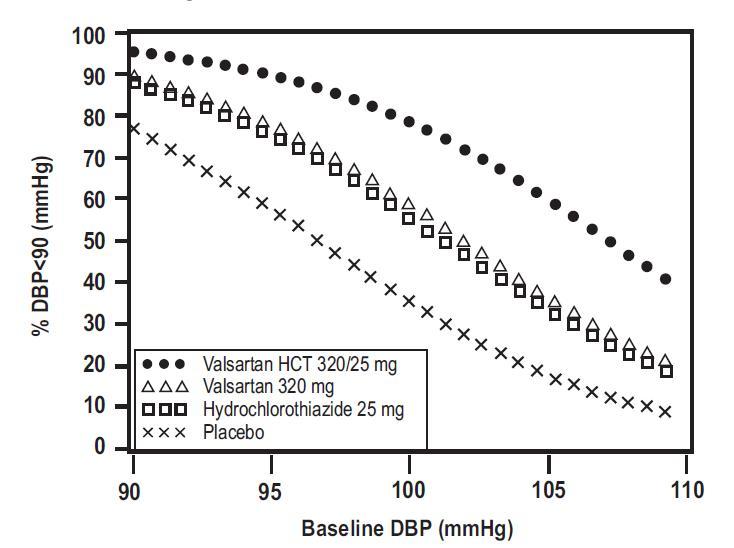
Figure 2: Probability of Achieving Diastolic Blood Pressure <90 mmHg at Week 8
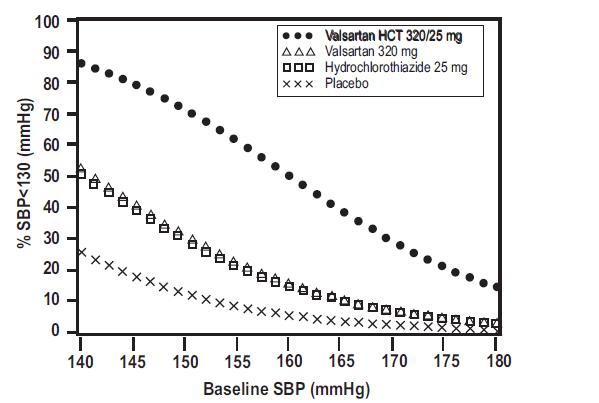
Figure 3: Probability of Achieving Systolic Blood Pressure <130 mmHg at Week 8
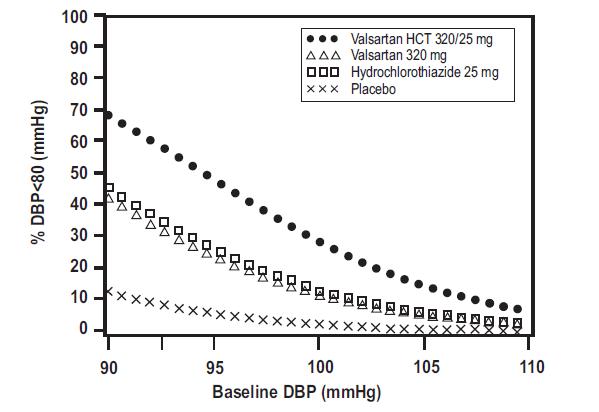
Figure 4: Probability of Achieving Diastolic Blood Pressure <80 mmHg at Week 8
Close
For example, a patient with a baseline blood pressure of 160/100 mmHg has about a 41% likelihood of achieving a goal of < 140 mmHg (systolic) and 60% likelihood of achieving < 90 mmHg (diastolic) on valsartan alone and the likelihood of achieving these goals on HCTZ alone is about 50% (systolic) or 57% (diastolic). The likelihood of achieving these goals on valsartan and hydrochlorothiazide tablets rises to about 84% (systolic) or 80% (diastolic). The likelihood of achieving these goals on placebo is about 23% (systolic) or 36% (diastolic). -
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - The usual starting dose is valsartan and hydrochlorothiazide tablets 160/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum ...
2.1 General Considerations
The usual starting dose is valsartan and hydrochlorothiazide tablets 160/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of one 320/25 tablet once daily as needed to control blood pressure [see Clinical Studies (14.2)]. Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
2.2 Add-On Therapy
A patient whose blood pressure is not adequately controlled with valsartan (or another ARB) alone or hydrochlorothiazide alone may be switched to combination therapy with valsartan and hydrochlorothiazide tablets.
A patient who experiences dose-limiting adverse reactions on either component alone may be switched to valsartan and hydrochlorothiazide tablets containing a lower dose of that component in combination with the other to achieve similar blood pressure reductions. The clinical response to valsartan and hydrochlorothiazide tablets should be subsequently evaluated and if blood pressure remains uncontrolled after 3 to 4 weeks of therapy, the dose may be titrated up to a maximum of 320/25 mg.
2.3 Replacement Therapy
Valsartan and hydrochlorothiazide tablets may be substituted for the titrated components.
2.4 Initial Therapy
Valsartan and hydrochlorothiazide tablets are not recommended as initial therapy in patients with intravascular volume depletion [see Warnings and Precautions (5.2)].
Close2.5 Use with Other Antihypertensive Drugs
Valsartan and hydrochlorothiazide tablets may be administered with other antihypertensive agents.
-
3 DOSAGE FORMS & STRENGTHS80/12.5 mg tablets, debossed with "L16" on one side and plain on other side - 160/12.5 mg tablets, debossed with "L17" on one side and plain on other side - 160/25 mg tablets, debossed with "L18" on ...
80/12.5 mg tablets, debossed with "L16" on one side and plain on other side
160/12.5 mg tablets, debossed with "L17" on one side and plain on other side
160/25 mg tablets, debossed with "L18" on one side and plain on other side
320/12.5 mg tablets, debossed with "L19" on one side and plain on other side
320/25 mg tablets, debossed with "L20" on one side and plain on other side
Close -
4 CONTRAINDICATIONSValsartan and hydrochlorothiazide tablets is contraindicated in patients who are hypersensitive to any component of this product. Because of the hydrochlorothiazide component, this product is ...
Valsartan and hydrochlorothiazide tablets is contraindicated in patients who are hypersensitive to any component of this product.
Close
Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
Do not coadminister aliskiren with valsartan and hydrochlorothiazide tablets in patients with diabetes [see Drug Interactions (7)]. -
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Valsartan and hydrochlorothiazide tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second ...
5.1 Fetal Toxicity
Valsartan and hydrochlorothiazide tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death.
Thiazides cross the placenta, and use of thiazides during pregnancy is associated with fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
When pregnancy is detected, discontinue valsartan and hydrochlorothiazide tablets as soon as possible [see Use in Specific Populations (8.1)].5.2 Hypotension in Volume- and/or Salt-Depleted Patients
Excessive reduction of blood pressure was rarely seen (0.7%) in patients with uncomplicated hypertension treated with valsartan and hydrochlorothiazide tablets in controlled trials. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur. This condition should be corrected prior to administration of valsartan and hydrochlorothiazide tablets, or the treatment should start under close medical supervision.
If hypotension occurs, place the patient in the supine position and, if necessary, give intravenous normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.3 Impaired Renal Function
Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on valsartan and hydrochlorothiazide tablets. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on valsartan and hydrochlorothiazide tablets [see Drug Interactions (7)].
5.4 Hypersensitivity Reaction
Hypersensitivity reactions to hydrochlorothiazide may occur in patients with or without a history of allergy or bronchial asthma, but are more likely in patients with such a history.
5.5 Systemic Lupus Erythematosus
Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
5.6 Lithium Interaction
Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of valsartan or thiazide diuretics. Monitor lithium levels in patients receiving valsartan and hydrochlorothiazide tablets and lithium [see Drug Interactions (7)].
5.7 Potassium Abnormalities
In the controlled trials of various doses of valsartan and hydrochlorothiazide tablets, the incidence of hypertensive patients who developed hypokalemia (serum potassium < 3.5 mEq/L) was 3.0%; the incidence of hyperkalemia (serum potassium > 5.7 mEq/L) was 0.4%.
Hydrochlorothiazide can cause hypokalemia and hyponatremia. Hypomagnesemia can result in hypokalemia which appears difficult to treat despite potassium repletion. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Monitor serum electrolytes periodically.
If hypokalemia is accompanied by clinical signs (e.g., muscular weakness, paresis, or ECG alterations), valsartan and hydrochlorothiazide tablets should be discontinued. Correction of hypokalemia and any coexisting hypomagnesemia is recommended prior to the initiation of thiazides.
Some patients with heart failure have developed increases in potassium with valsartan therapy. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of the diuretic and/or valsartan may be required [see Adverse Reactions (6.1)].
5.8 Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Close5.9 Metabolic Disturbances
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hydrochlorothiazide may raise the serum uric acid level due to reduced clearance of uric acid and may cause or exacerbate hyperuricemia and precipitate gout in susceptible patients.
Hydrochlorothiazide decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels in patients with hypercalcemia receiving valsartan and hydrochlorothiazide tablets.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly ...
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Hypertension
Valsartan and hydrochlorothiazide tablets have been evaluated for safety in more than 5,700 patients, including over 990 treated for over 6 months, and over 370 for over 1 year. Adverse experiences have generally been mild and transient in nature and have only infrequently required discontinuation of therapy. The overall incidence of adverse reactions with valsartan and hydrochlorothiazide tablets was comparable to placebo.
The overall frequency of adverse reactions was neither dose-related nor related to gender, age, or race. In controlled clinical trials, discontinuation of therapy due to side effects was required in 2.3% of valsartan-hydrochlorothiazide patients and 3.1% of placebo patients. The most common reasons for discontinuation of therapy with valsartan and hydrochlorothiazide tablets were headache and dizziness.
The only adverse reaction that occurred in controlled clinical trials in at least 2% of patients treated with valsartan and hydrochlorothiazide tablets and at a higher incidence in valsartan-hydrochlorothiazide (n=4372) than placebo (n=262) patients was nasopharyngitis (2.4% vs. 1.9%).
Dose-related orthostatic effects were seen in fewer than 1% of patients. In individual trials, a dose-related increase in the incidence of dizziness was observed in patients treated with valsartan and hydrochlorothiazide tablets.
Initial Therapy-Hypertension
In a clinical study in patients with severe hypertension (diastolic blood pressure ≥ 110 mmHg and systolic blood pressure ≥ 140 mmHg), the overall pattern of adverse reactions reported through 6 weeks of follow-up was similar in patients treated with valsartan and hydrochlorothiazide tablets as initial therapy and in patients treated with valsartan as initial therapy. Comparing the groups treated with valsartan and hydrochlorothiazide tablets (force-titrated to 320/25 mg) and valsartan (force-titrated to 320 mg), dizziness was observed in 6% and 2% of patients, respectively. Hypotension was observed in 1% of those patients receiving valsartan and hydrochlorothiazide tablets and 0% of patients receiving valsartan. There were no reported cases of syncope in either treatment group. Laboratory changes with valsartan and hydrochlorothiazide tablets as initial therapy in patients with severe hypertension were similar to those reported with valsartan and hydrochlorothiazide tablets in patients with less severe hypertension [see Clinical Studies (14.2), Drug Interactions (7)].
Valsartan: In trials in which valsartan was compared to an ACE inhibitor with or without placebo, the incidence of dry cough was significantly greater in the ACE inhibitor group (7.9%) than in the groups who received valsartan (2.6%) or placebo (1.5%). In a 129-patient trial limited to patients who had had dry cough when they had previously received ACE inhibitors, the incidences of cough in patients who received valsartan, hydrochlorothiazide, or lisinopril were 20%, 19%, 69% respectively (p < 0.001).
Close6.2 Postmarketing Experience
The following additional adverse reactions have been reported in valsartan or valsartan/ hydrochlorothiazide postmarketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: Angioedema has been reported. Some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Valsartan and hydrochlorothiazide tablets should not be re-administered to patients who have had angioedema.
Digestive: Elevated liver enzymes and reports of hepatitis
Musculoskeletal: Rhabdomyolysis
Renal: Impaired renal function
Dermatologic: Alopecia, bullous dermatitis
Vascular: Vasculitis
Nervous System: Syncope
Hydrochlorothiazide:
The following additional adverse reactions have been reported in postmarketing experience with hydrochlorothiazide:
Acute renal failure, renal disorder, aplastic anemia, erythema multiforme, pyrexia, muscle spasm, asthenia, acute angle-closure glaucoma, bone marrow failure, worsening of diabetes control, hypokalemia, blood lipids increased, hyponatremia, hypomagnesemia, hypercalcemia, hypochloremic alkalosis, impotence, and visual impairment.
Pathological changes in the parathyroid gland of patients with hypercalcemia and hypophosphatemia have been observed in a few patients on prolonged thiazide therapy. If hypercalcemia occurs, further diagnostic evaluation is necessary.
Non-melanoma Skin Cancer: Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥ 50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year. -
7 DRUG INTERACTIONSValsartan-Hydrochlorothiazide: Lithium: Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II ...
Valsartan-Hydrochlorothiazide:
Lithium: Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists or thiazides. Monitor lithium levels in patients taking valsartan and hydrochlorothiazide tablets.
Valsartan:
Agents Increasing Serum Potassium: Concomitant use of valsartan with other agents that block the renin-angiotensin system, potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, salt substitutes containing potassium or other drugs that may increase potassium levels (e.g., heparin) may lead to increases in serum potassium and in heart failure patients to increases in serum creatinine. If co-medication is considered necessary, monitoring of serum potassium is advisable.
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including valsartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving valsartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including valsartan, may be attenuated by NSAIDs including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on valsartan and other agents that affect the RAS.
Do not coadminister aliskiren with valsartan in patients with diabetes. Avoid use of aliskiren with valsartan in patients with renal impairment (GFR < 60 mL/min).
Hydrochlorothiazide:
When administered concurrently, the following drugs may interact with thiazide diuretics:
Antidiabetic Drugs (oral agents and insulin) - Dosage adjustment of the antidiabetic drug may be required.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs and COX-2 selective inhibitors) - When valsartan and hydrochlorothiazide tablets and nonsteroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
Carbamazepine - May lead to symptomatic hyponatremia.
Ion exchange resins: Staggering the dosage of hydrochlorothiazide and ion exchange resins (e.g., cholestyramine, colestipol) such that hydrochlorothiazide is administered at least 4 hours before or 4 to 6 hours after the administration of resins would potentially minimize the interaction [see Clinical Pharmacology (12.3)].
Cyclosporine: Concomitant treatment with cyclosporine may increase the risk of hyperuricemia and gout-type complications.
Close -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Valsartan and hydrochlorothiazide tablet can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during ...
8.1 Pregnancy
Risk Summary
Valsartan and hydrochlorothiazide tablet can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Published reports include cases of anhydramnios and oligohydramnios in pregnant women treated with valsartan (see Clinical Considerations).
When pregnancy is detected discontinue valsartan and hydrochlorothiazide tablet as soon as possible.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Valsartan
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension and death.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment. Closely observe neonates with histories of in utero exposure to valsartan and hydrochlorothiazide tablet for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to valsartan and hydrochlorothiazide tablet, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
Hydrochlorothiazide
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma. Hydrochlorothiazide, like other diuretics, can cause placental hypoperfusion. It accumulates in the amniotic fluid, with reported concentrations up to 19 times higher than in umbilical vein plasma. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice or thrombocytopenia. Since they do not prevent or alter the course of EPH (Edema, Proteinuria, Hypertension) gestosis (pre-eclampsia), these drugs should not be used to treat hypertension in pregnant women. The use of hydrochlorothiazide for other indications (e.g., heart disease) in pregnancy should be avoided.
Data
Animal Data
Valsartan plus Hydrochlorothiazide
There was no evidence of teratogenicity in mice, rats, or rabbits treated orally with valsartan at doses of up to 600, 100, and 10 mg/kg/day [9, 3.5 and 0.5 times the maximum recommended human dose (MRHD)], respectively, in combination with hydrochlorothiazide at doses up to 188, 31, and 3 mg/kg/day (38, 13 and 2 times the MRHD).Fetotoxicity was observed in association with maternal toxicity in rats at valsartan/hydrochlorothiazide doses of ≥ 200/63 mg/kg/day and in rabbits at valsartan/hydrochlorothiazide doses of 10/3 mg/kg/day. Evidence of fetotoxicity in rats consisted of decreased fetal weight and fetal variations of sternebrae, vertebrae, ribs, and/or renal papillae. Evidence of fetotoxicity in rabbits included increased numbers of late resorptions with resultant increases in total resorptions, post-implantation losses, and decreased number of live fetuses.
8.2 Lactation
Risk Summary
There is limited information regarding the presence of valsartan and hydrochlorothiazide tablet in human milk, the effects on the breastfed infant, or the effects on milk production. Valsartan is present in rat milk. Hydrochlorothiazide is present in human breast milk. Because of the potential for serious adverse reactions in breastfed infants, advise a nursing woman that breastfeeding is not recommended during treatment with valsartan and hydrochlorothiazide tablet.Data
Valsartan was detected in the milk of lactating rats 15 minutes after oral administration of a 3 mg/kg dose.8.4 Pediatric Use
Safety and effectiveness of valsartan and hydrochlorothiazide tablet in pediatric patients have not been established.
8.5 Geriatric Use
In the controlled clinical trials of valsartan and hydrochlorothiazide tablets, 764 (17.5%) patients treated with valsartan-hydrochlorothiazide were ≥ 65 years and 118 (2.7%) were ≥ 75 years. No overall difference in the efficacy or safety of valsartan-hydrochlorothiazide was observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Safety and effectiveness of valsartan and hydrochlorothiazide tablets in patients with severe renal impairment (CrCl ≤ 30 mL/min) have not been established. No dose adjustment is required in patients with mild (CrCl 60 to 90 mL/min) or moderate (CrCl 30 to 60mL/min) renal impairment.
Close8.7 Hepatic Impairment
Valsartan
No dose adjustment is necessary for patients with mild-to-moderate liver disease. No dosing recommendations can be provided for patients with severe liver disease.
Hydrochlorothiazide
Minor alterations of fluid and electrolyte balance may precipitate hepatic coma in patients with impaired hepatic function or progressive liver disease.
-
10 OVERDOSAGELimited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic ...
Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. Depressed level of consciousness, circulatory collapse and shock have been reported. If symptomatic hypotension should occur institute supportive treatment.
Close
Valsartan is not removed from the plasma by dialysis.
The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The most common signs and symptoms observed in patients are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
In rats and marmosets, single oral doses of valsartan up to 1524 and 762 mg/kg in combination with hydrochlorothiazide at doses up to 476 and 238 mg/kg, respectively, did not show any adverse treatment-related effects. These no adverse effect doses in rats and marmosets, respectively, represent 46.5 and 23 times the MRHD of valsartan and 188 and 113 times the MRHD of hydrochlorothiazide on a mg/m2 basis. (Calculations assume an oral dose of 320 mg/day valsartan in combination with 25 mg/day hydrochlorothiazide and a 60-kg patient.)
Valsartan was without grossly observable adverse effects at single oral doses up to 2000 mg/kg in rats and up to 1000 mg/kg in marmosets, except for salivation and diarrhea in the rat and vomiting in the marmoset at the highest dose (60 and 31 times, respectively, the MRHD on a mg/m2 basis) (Calculations assume an oral dose of 320 mg/day and a 60-kg patient).
The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats, which represents 2027 and 4054 times, respectively, the MRHD on a mg/m2 basis. (Calculations assume an oral dose of 25 mg/day and a 60-kg patient). -
11 DESCRIPTIONValsartan and hydrochlorothiazide tablets, USP are a combination of valsartan, an orally active, specific angiotensin II receptor blocker (ARB) acting on the AT1 receptor subtype, and ...
Valsartan and hydrochlorothiazide tablets, USP are a combination of valsartan, an orally active, specific angiotensin II receptor blocker (ARB) acting on the AT1 receptor subtype, and hydrochlorothiazide, a diuretic.
Valsartan, a nonpeptide molecule, is chemically described as N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-Valine. Its empirical formula is C24H29N5O3, its molecular weight is 435.5, and its structural formula is:
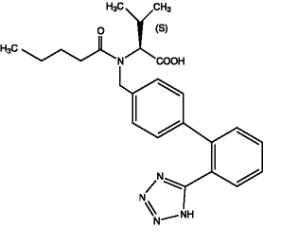
Valsartan is a white to practically white fine powder. It is soluble in ethanol and methanol and slightly soluble in water.
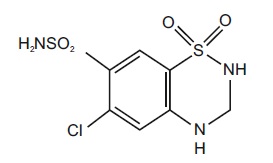
Hydrochlorothiazide, USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water; freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; and insoluble in ether, in chloroform, and in dilute mineral acids. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide.
Hydrochlorothiazide is a thiazide diuretic. Its empirical formula is C7H8ClN3O4S2, its molecular weight is 297.73, and its structural formula is:
Valsartan and hydrochlorothiazide tablets, USP, are formulated for oral administration to contain valsartan and hydrochlorothiazide, USP 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg and 320/25 mg. The inactive ingredients of the tablets are colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides, magnesium stearate, microcrystalline cellulose, polyethylene glycol, talc, and titanium dioxide.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent ...
12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Valsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Valsartan has much greater affinity (about 20,000-fold) for the AT1 receptor than for the AT2 receptor. The primary metabolite of valsartan is essentially inactive with an affinity for the AT1 receptor about one 200th that of valsartan itself.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because valsartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Valsartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of valsartan on blood pressure.
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so coadministration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is unknown.
12.2 Pharmacodynamics
Valsartan: Valsartan inhibits the pressor effect of angiotensin II infusions. An oral dose of 80 mg inhibits the pressor effect by about 80% at peak with approximately 30% inhibition persisting for 24 hours. No information on the effect of larger doses is available.
Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin and consequent rise in angiotensin II plasma concentration in hypertensive patients. Minimal decreases in plasma aldosterone were observed after administration of valsartan; very little effect on serum potassium was observed.
Hydrochlorothiazide: After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Pharmacodynamic Drug Interactions
Hydrochlorothiazide:
Alcohol, barbiturates, or narcotics: Potentiation of orthostatic hypotension may occur.
Skeletal muscle relaxants: Possible increased responsiveness to muscle relaxants such as curare derivatives.
Digitalis glycosides: Thiazide-induced hypokalemia or hypomagnesemia may predispose the patient to digoxin toxicity.
Close12.3 Pharmacokinetics
Valsartan: Valsartan peak plasma concentration is reached 2 to 4 hours after dosing. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. Absolute bioavailability for the capsule formulation is about 25% (range 10% to 35%). Food decreases the exposure (as measured by area under the curve [AUC]) to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%. AUC and Cmax values of valsartan increase approximately linearly with increasing dose over the clinical dosing range. Valsartan does not accumulate appreciably in plasma following repeated administration.
Hydrochlorothiazide: The estimated absolute bioavailability of hydrochlorothiazide after oral administration is about 70%. Peak plasma hydrochlorothiazide concentrations (Cmax) are reached within 2 to 5 hours after oral administration. There is no clinically significant effect of food on the bioavailability of hydrochlorothiazide.
Hydrochlorothiazide binds to albumin (40% to 70%) and distributes into erythrocytes. Following oral administration, plasma hydrochlorothiazide concentrations decline bi-exponentially, with a mean distribution half-life of about 2 hours and an elimination half-life of about 10 hours.
Valsartan and hydrochlorothiazide tablets: Valsartan and hydrochlorothiazide tablets may be administered with or without food.
Distribution
Valsartan: The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Valsartan is highly bound to serum proteins (95%), mainly serum albumin.
Metabolism
Valsartan: The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism.
Hydrochlorothiazide: Is not metabolized.
Excretion
Valsartan: Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites.
Following intravenous administration, plasma clearance of valsartan is about 2 L/h and its renal clearance is 0.62 L/h (about 30% of total clearance).
Hydrochlorothiazide:
About 70% of an orally administered dose of hydrochlorothiazide is eliminated in the urine as unchanged drug.
Specific Populations
Geriatric: Exposure (measured by AUC) to valsartan is higher by 70% and the half-life is longer by 35% in the elderly than in the young. A limited amount of data suggest that the systemic clearance of hydrochlorothiazide is reduced in both healthy and hypertensive elderly subjects compared to young healthy volunteers.
Gender: Pharmacokinetics of valsartan do not differ significantly between males and females.
Race: Pharmacokinetic differences due to race have not been studied.
Renal Insufficiency: There is no apparent correlation between renal function (measured by creatinine clearance) and exposure (measured by AUC) to valsartan in patients with different degrees of renal impairment. Valsartan has not been studied in patients with severe impairment of renal function (creatinine clearance < 10 mL/min). Valsartan is not removed from the plasma by hemodialysis.
In a study in individuals with impaired renal function, the mean elimination half-life of hydrochlorothiazide was doubled in individuals with mild/moderate renal impairment (30 < CrCl < 90 mL/min) and tripled in severe renal impairment (CrCl ≤ 30 mL/min), compared to individuals with normal renal function (CrCl > 90 mL/min)[see Use in Specific Populations (8.6)].
Hepatic Insufficiency: On average, patients with mild-to-moderate chronic liver disease have twice the exposure (measured by AUC values) to valsartan of healthy volunteers (matched by age, sex, and weight)[see Use in Specific Populations (8.7)].
Drug Interactions
Valsartan
No clinically significant pharmacokinetic interactions were observed when valsartan was coadministered with amlodipine, atenolol, cimetidine, digoxin, furosemide, glyburide, hydrochlorothiazide, or indomethacin. The valsartan-atenolol combination was more antihypertensive than either component, but it did not lower the heart rate more than atenolol alone.Coadministration of valsartan and warfarin did not change the pharmacokinetics of valsartan or the time-course of the anticoagulant properties of warfarin.
Transporters: The results from an in vitro study with human liver tissue indicate that valsartan is a substrate of the hepatic uptake transporter OATP1B1 and the hepatic efflux transporter MRP2. Coadministration of inhibitors of the uptake transporter (rifampin, cyclosporine) or efflux transporter (ritonavir) may increase the systemic exposure to valsartan.
Hydrochlorothiazide:
Drugs that alter gastrointestinal motility: The bioavailability of thiazide-type diuretics may be increased by anticholinergic agents (e.g., atropine, biperiden), apparently due to a decrease in gastrointestinal motility and the stomach emptying rate. Conversely, pro-kinetic drugs may decrease the bioavailability of thiazide diuretics.
Cholestyramine: In a dedicated drug interaction study, administration of cholestyramine 2 hours before hydrochlorothiazide resulted in a 70% reduction in exposure to hydrochlorothiazide. Further, administration of hydrochlorothiazide 2 hours before cholestyramine resulted in 35% reduction in exposure to hydrochlorothiazide.
Antineoplastic agents (e.g., cyclophosphamide, methotrexate): Concomitant use of thiazide diuretics may reduce renal excretion of cytotoxic agents and enhance their myelosuppressive effects. -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Valsartan-Hydrochlorothiazide: No carcinogenicity, mutagenicity, or fertility studies have been conducted with the combination of ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Valsartan-Hydrochlorothiazide: No carcinogenicity, mutagenicity, or fertility studies have been conducted with the combination of valsartan and hydrochlorothiazide. However, these studies have been conducted for valsartan as well as hydrochlorothiazide alone. Based on the preclinical safety and human pharmacokinetic studies, there is no indication of any adverse interaction between valsartan and hydrochlorothiazide.
Valsartan: There was no evidence of carcinogenicity when valsartan was administered in the diet to mice and rats for up to 2 years at doses up to 160 and 200 mg/kg/day, respectively. These doses in mice and rats are about 2.6 and 6 times, respectively, the MRHD on a mg/m2 basis (Calculations assume an oral dose of 320 mg/day and a 60-kg patient).
Mutagenicity assays did not reveal any valsartan-related effects at either the gene or chromosome level. These assays included bacterial mutagenicity tests with Salmonella (Ames) and E. coli; a gene mutation test with Chinese hamster V79 cells; a cytogenetic test with Chinese hamster ovary cells; and a rat micronucleus test.
Valsartan had no adverse effects on the reproductive performance of male or female rats at oral doses up to 200 mg/kg/day. This dose is about 6 times the MRHD on a mg/m2 basis (Calculations assume an oral dose of 320 mg/day and a 60-kg patient).
Hydrochlorothiazide: Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 mcg/mL, and in the Aspergillus Nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to mating and throughout gestation. These doses of hydrochlorothiazide in mice and rats represent 19 and 1.5 times, respectively, the MRHD on a mg/m2 basis. (Calculations assume an oral dose of 25 mg/day and a 60-kg patient.)
-
14 CLINICAL STUDIES14.1 Hypertension - Valsartan-Hydrochlorothiazide: In controlled clinical trials including over 7600 patients, 4372 patients were exposed to valsartan (80, 160, and 320 mg) and concomitant ...
14.1 Hypertension
Valsartan-Hydrochlorothiazide: In controlled clinical trials including over 7600 patients, 4372 patients were exposed to valsartan (80, 160, and 320 mg) and concomitant hydrochlorothiazide (12.5 and 25 mg). Two factorial trials compared various combinations of 80/12.5 mg, 80/25 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg, and 320/25 mg with their respective components and placebo. The combination of valsartan and hydrochlorothiazide resulted in additive placebo-adjusted decreases in systolic and diastolic blood pressure at trough of 14-21/8-11 mmHg at 80/12.5 mg to 320/25 mg, compared to 7-10/4-5 mmHg for valsartan 80 mg to 320 mg, and 5-11/2-5 mmHg for hydrochlorothiazide 12.5 mg to 25 mg alone.
Three other controlled trials investigated the addition of hydrochlorothiazide to patients who did not respond adequately to valsartan 80 mg to valsartan 320 mg, resulted in the additional lowering of systolic and diastolic blood pressure by approximately 4-12/2-5 mmHg.
The maximal antihypertensive effect was attained 4 weeks after the initiation of therapy, the first time point at which blood pressure was measured in these trials.
In long-term follow-up studies (without placebo control), the effect of the combination of valsartan and hydrochlorothiazide appeared to be maintained for up to 2 years. The antihypertensive effect is independent of age or gender. The overall response to the combination was similar for black and non-black patients.
There was essentially no change in heart rate in patients treated with the combination of valsartan and hydrochlorothiazide in controlled trials.
There are no trials of the valsartan and hydrochlorothiazide combination tablet demonstrating reductions in cardiovascular risk in patients with hypertension, but the hydrochlorothiazide component and several ARBs, which are the same pharmacological class as the valsartan component, have demonstrated such benefits.
Valsartan: The antihypertensive effects of valsartan were demonstrated principally in 7 placebo-controlled, 4- to 12-week trials (1 in patients over 65 years) of dosages from 10 to 320 mg/day in patients with baseline diastolic blood pressures of 95-115 mmHg. The studies allowed comparison of once-daily and twice-daily regimens of 160 mg/day; comparison of peak and trough effects; comparison (in pooled data) of response by gender, age, and race; and evaluation of incremental effects of hydrochlorothiazide.
Administration of valsartan to patients with essential hypertension results in a significant reduction of sitting, supine, and standing systolic and diastolic blood pressure, usually with little or no orthostatic change.
In most patients, after administration of a single oral dose, onset of antihypertensive activity occurs at approximately 2 hours, and maximum reduction of blood pressure is achieved within 6 hours. The antihypertensive effect persists for 24 hours after dosing, but there is a decrease from peak effect at lower doses (40 mg) presumably reflecting loss of inhibition of angiotensin II. At higher doses, however (160 mg), there is little difference in peak and trough effect. During repeated dosing, the reduction in blood pressure with any dose is substantially present within 2 weeks, and maximal reduction is generally attained after 4 weeks. In long-term follow-up studies (without placebo control), the effect of valsartan appeared to be maintained for up to 2 years. The antihypertensive effect is independent of age, gender or race. The latter finding regarding race is based on pooled data and should be viewed with caution, because antihypertensive drugs that affect the renin-angiotensin system (that is, ACE inhibitors and angiotensin II blockers) have generally been found to be less effective in low-renin hypertensives (frequently blacks) than in high-renin hypertensives (frequently whites). In pooled, randomized, controlled trials of valsartan that included a total of 140 blacks and 830 whites, valsartan and an ACE-inhibitor control were generally at least as effective in blacks as whites. The explanation for this difference from previous findings is unclear.
Abrupt withdrawal of valsartan has not been associated with a rapid increase in blood pressure.
The 7 studies of valsartan monotherapy included over 2000 patients randomized to various doses of valsartan and about 800 patients randomized to placebo. Doses below 80 mg were not consistently distinguished from those of placebo at trough, but doses of 80, 160 and 320 mg produced dose-related decreases in systolic and diastolic blood pressure, with the difference from placebo of approximately 6-9/3-5 mmHg at 80 to 160 mg and 9/6 mmHg at 320 mg.
Patients with an inadequate response to 80 mg once daily were titrated to either 160 mg once daily or 80 mg twice daily, which resulted in a similar response in both groups.
In another 4-week study, 1876 patients randomized to valsartan 320 mg once daily had an incremental blood pressure reduction 3/1 mmHg lower than did 1900 patients randomized to valsartan 160 mg once daily.
In controlled trials, the antihypertensive effect of once daily valsartan 80 mg was similar to that of once daily enalapril 20 mg or once daily lisinopril 10 mg.
There was essentially no change in heart rate in valsartan-treated patients in controlled trials.Close14.2 Initial Therapy - Hypertension
The safety and efficacy of valsartan and hydrochlorothiazide tablets as initial therapy for patients with severe hypertension (defined as a sitting diastolic blood pressure ≥ 110 mmHg and systolic blood pressure ≥ 140 mmHg off all antihypertensive therapy) were studied in a 6-week multicenter, randomized, double-blind study. Patients were randomized to either valsartan and hydrochlorothiazide tablets (valsartan and hydrochlorothiazide 160/12.5 mg once daily) or to valsartan (160 mg once daily) and followed for blood pressure response. Patients were force-titrated at 2-week intervals. Patients on combination therapy were subsequently titrated to 160/25 mg followed by 320/25 mg valsartan/hydrochlorothiazide. Patients on monotherapy were subsequently titrated to 320 mg valsartan followed by a titration to 320 mg valsartan to maintain the blind.
The study randomized 608 patients, including 261 (43%) females, 147 (24%) blacks, and 75 (12%) ≥ 65 years of age. The mean blood pressure at baseline for the total population was 168/112 mmHg. The mean age was 52 years. After 4 weeks of therapy, reductions in systolic and diastolic blood pressure were 9/5 mmHg greater in the group treated with valsartan and hydrochlorothiazide tablets compared to valsartan. Similar trends were seen when the patients were grouped according to gender, race, or age. -
16 HOW SUPPLIED/STORAGE AND HANDLINGValsartan and hydrochlorothiazide tablets, USP are available as non-scored tablets containing valsartan/hydrochlorothiazide 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg and 320/25 mg. Strengths ...
Valsartan and hydrochlorothiazide tablets, USP are available as non-scored tablets containing valsartan/hydrochlorothiazide 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg and 320/25 mg. Strengths are available as follows.
80/12.5 mg Tablet - Light orange, oval shaped biconvex film coated tablets debossed with "L16" on one side and plain on other side.
Bottles of 90 NDC 33342-074-10
Bottles of 1000 NDC 33342-074-44
160/12.5 mg Tablet - Dark red, oval shaped biconvex film coated tablets debossed with "L17" on one side and plain on other side.
Bottles of 90 NDC 33342-075-10
Bottles of 500 NDC 33342-075-15
160/25 mg Tablet - Brown orange, oval shaped biconvex film coated tablets debossed with "L18" on one side and plain on other side.
Bottles of 90 NDC 33342-076-10
Bottles of 500 NDC 33342-076-15
320/12.5 mg Tablet - Pink, oval shaped biconvex film coated tablets debossed with "L19" on one side and plain on other side.
Bottles of 90 NDC 33342-077-10
Bottles of 500 NDC 33342-077-15
320/25 mg Tablet - Yellow, oval shaped biconvex film coated tablets debossed with "L20" on one side and plain on other side.
Bottles of 90 NDC 33342-078-10
Bottles of 500 NDC 33342-078-15
Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
Close -
17 PATIENT COUNSELING INFORMATIONInformation for Patients - Advise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of ...
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy: Advise female patients of childbearing age about the consequences of exposure to valsartan and hydrochlorothiazide tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Lactation: Advise women not to breastfeed during treatment with valsartan and hydrochlorothiazide tablets [see Use in Specific Populations (8.2)].
Symptomatic Hypotension: Advise patients that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to their healthcare provider. Tell patients that if syncope occurs, to discontinue valsartan and hydrochlorothiazide tablets until the physician has been consulted. Caution all patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope [see Warnings and Precautions (5.2)].
Potassium Supplements: Advise patients not to use salt substitutes without consulting their healthcare provider [see Drug Interactions (7)].
Non-melanoma Skin Cancer: Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Manufactured for:
Macleods Pharma USA, Inc.,
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceuticals Ltd.
Daman (U.T.) INDIA
OR
Manufactured by :
Macleods Pharmaceuticals Ltd.
Baddi, Himachal Pradesh, INDIA
Revised: 11/2022
Close -
SPL PATIENT PACKAGE INSERTFDA-Approved Patient Labeling - PATIENT INFORMATION - Valsartan and Hydrochlorothiazide Tablets - (val sar' tan and hye'' droe klor'' oh thye' a zide) Read the Patient Information that ...
FDA-Approved Patient Labeling
PATIENT INFORMATION
Valsartan and Hydrochlorothiazide Tablets
(val sar' tan and hye'' droe klor'' oh thye' a zide)
Read the Patient Information that comes with valsartan and hydrochlorothiazide tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment. If you have any questions about valsartan and hydrochlorothiazide tablets, ask your doctor or pharmacist.
What is the most important information I should know about valsartan and hydrochlorothiazide tablets?
Valsartan and hydrochlorothiazide tablets can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking valsartan and hydrochlorothiazide tablets, tell your doctor right away.
What are valsartan and hydrochlorothiazide tablets?
Valsartan and hydrochlorothiazide tablets contain 2 prescription medicines:
1. valsartan, an angiotensin receptor blocker (ARB)
2. hydrochlorothiazide (HCTZ), a water pill (diuretic)
Valsartan and hydrochlorothiazide tablets may be used to lower high blood pressure (hypertension) in adults-
• when 1 medicine to lower your high blood pressure is not enough.
• as the first medicine to lower high blood pressure if your doctor decides you are likely to need more than 1 medicine.
Valsartan and hydrochlorothiazide tablets have not been studied in children under 18 years of age.
Who should not take valsartan and hydrochlorothiazide tablets?
Do not take valsartan and hydrochlorothiazide tablets if you:
• are allergic to any of the ingredients in valsartan and hydrochlorothiazide tablets. See the end of this leaflet for a complete list of ingredients in valsartan and hydrochlorothiazide tablets.
• make less urine due to kidney problems.
• are allergic to medicines that contain sulfonamides.
What should I tell my doctor before taking valsartan and hydrochlorothiazide tablets?
Tell your doctor about all your medical conditions including if you:
• are pregnant or plan to become pregnant. See "What is the most important information I should know about valsartan and hydrochlorothiazide tablets?"
• are breastfeeding. Valsartan and hydrochlorothiazide passes into breast milk. It is not known if valsartan and hydrochlorothiazide effects your breastfed baby or milk production. Do not breastfeed while you are taking valsartan and hydrochlorothiazide tablets.
• have liver problems
• have kidney problems
• have or had gallstones
• have Lupus
• have low levels of potassium (with or without symptoms such as muscle weakness, muscle spasms, abnormal heart rhythm) or magnesium in your blood
• have high levels of calcium in your blood (with or without symptoms such as nausea, vomiting, constipation, stomach pain, frequent urination, thirst, muscle weakness and twitching)
• have high levels of uric acid in the blood
• have ever had a reaction called angioedema, to another blood pressure medication. Angioedema causes swelling of the face, lips, tongue, throat and may cause difficulty breathing
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Some of your other medicines and valsartan and hydrochlorothiazide tablets could affect each other, causing serious side effects. Especially, tell your doctor if you take:
• other medicines for high blood pressure or a heart problem
• water pills (diuretics)
• potassium supplements. Your doctor may check the amount of potassium in your blood periodically.
• a salt substitute. Your doctor may check the amount of potassium in your blood periodically.
• antidiabetic medicines including insulin
• narcotic pain medicines
• sleeping pills
• lithium, a medicine used in some types of depression (Eskalith®, Lithobid®, Lithium Carbonate, Lithium Citrate)
• aspirin or other medicines called non-steroidal anti-inflammatory drugs (NSAIDs), like ibuprofen or naproxen
• digoxin or other digitalis glycosides (a heart medicine)
• muscle relaxants (medicines used during operations)
• certain cancer medicines, like cyclophosphamide or methotrexate
• certain antibiotics (rifamycin group), a drug used to protect against transplant rejection (cyclosporine) or an antiretroviral drug used to treat HIV/AIDS infection (ritonavir). These drugs may increase the effect of valsartan.
Ask your doctor if you are not sure if you are taking one of these medicines.
Know the medicines you take. Keep a list of your medicines with you to show to your doctor and pharmacist when a new medicine is prescribed. Talk to your doctor or pharmacist before you start taking any new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take valsartan and hydrochlorothiazide tablets?
• Take valsartan and hydrochlorothiazide tablets exactly as prescribed by your doctor. Your doctor may change your dose if needed.
• Take valsartan and hydrochlorothiazide tablets once each day.
• Valsartan and hydrochlorothiazide tablets can be taken with or without food.
• If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
• If you take too much valsartan and hydrochlorothiazide tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room.
What should I avoid while taking valsartan and hydrochlorothiazide tablets?
You should not take valsartan and hydrochlorothiazide tablets during pregnancy. See "What is the most important information I should know about valsartan and hydrochlorothiazide tablets?"
What are the possible side effects of valsartan and hydrochlorothiazide tablets?
Valsartan and hydrochlorothiazide tablets may cause serious side effects including:
• Harm to an unborn baby causing injury and even death. See "What is the most important information I should know about valsartan and hydrochlorothiazide tablets?"
• Low blood pressure (hypotension). Low blood pressure is most likely to happen if you:
o take water pills
o are on a low-salt diet
o get dialysis treatments
o have heart problems
o get sick with vomiting or diarrhea
o drink alcohol
Lie down if you feel faint or dizzy. Call your doctor right away.
• Allergic reactions. People with and without allergy problems or asthma who take valsartan and hydrochlorothiazide tablets may get allergic reactions.
• Worsening of Lupus. Hydrochlorothiazide, one of the medicines in valsartan and hydrochlorothiazide tablets may cause Lupus to become active or worse.
• Fluid and electrolyte (salt) problems. Tell your doctor about any of the following signs and symptoms of fluid and electrolyte problems:
• dry mouth
• thirst
• lack of energy (lethargic)
• weakness
• drowsiness
• restlessness
• confusion
• seizures
• muscle pain or cramps
• muscle fatigue
• very low urine output
• fast heartbeat
• nausea and vomiting
• Kidney problems. Kidney problems may become worse in people that already have kidney disease. Some people will have changes on blood tests for kidney function and may need a lower dose of valsartan and hydrochlorothiazide tablets. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain. If you have heart failure, your doctor should check your kidney function before prescribing valsartan and hydrochlorothiazide tablets.
• Skin rash. Call your doctor right away if you have an unusual skin rash.
• Eye problems. One of the medicines in valsartan and hydrochlorothiazide tablets can cause eye problems that may lead to vision loss. Symptoms of eye problems can happen within hours to weeks of starting valsartan and hydrochlorothiazide tablets. Tell your doctor right away if you have:
• decrease in vision
• eye pain
Protect your skin from the sun and undergo regular skin cancer screening, as one of the medicines in valsartan and hydrochlorothiazide tablets may cause non-melanoma skin cancer.
Other side effects were generally mild and brief. They generally have not caused patients to stop taking valsartan and hydrochlorothiazide tablets.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of valsartan and hydrochlorothiazide tablets. For a complete list, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store valsartan and hydrochlorothiazide tablets?
• Store valsartan and hydrochlorothiazide tablets at room temperature between 59ºF to 86ºF (15ºC to 30ºC).
• Keep valsartan and hydrochlorothiazide tablets in a closed container in a dry place.
Keep valsartan and hydrochlorothiazide tablets and all medicines out of the reach of children.
General information about valsartan and hydrochlorothiazide tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use valsartan and hydrochlorothiazide tablets for a condition for which it was not prescribed. Do not give valsartan and hydrochlorothiazide tablets to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about valsartan and hydrochlorothiazide tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about valsartan and hydrochlorothiazide tablets that is written for health professionals. For more information about valsartan and hydrochlorothiazide tablets, call 1-888-943-3210 or 1-855-926-3384.
What are the ingredients in valsartan and hydrochlorothiazide tablets?
Active ingredients: Valsartan and hydrochlorothiazide
Inactive ingredients: colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides, magnesium stearate, microcrystalline cellulose, polyethylene glycol, talc, and titanium dioxide.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Valsartan and hydrochlorothiazide tablets can help your blood vessels relax and reduce the amount of water in your body so your blood pressure is lower. Medicines that lower blood pressure lower your risk of having a stroke or heart attack.
High blood pressure makes the heart work harder to pump blood throughout the body and causes damage to the blood vessels. If high blood pressure is not treated, it can lead to stroke, heart attack, heart failure, kidney failure, and vision problems.
Eskalith® and Lithobid® are registered trademarks of Noven Pharmaceuticals, Inc.
Manufactured for:
Macleods Pharma USA, Inc.
Princeton, NJ 08540Manufactured by:
Macleods Pharmaceuticals Ltd.
Daman (U.T.) INDIAOR
Manufactured by :
Macleods Pharmaceuticals Ltd.
Baddi, Himachal Pradesh, INDIA
Revised: 11/2022
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELValsartan and Hydrochlorothiazide Tablets, 80/12.5 mg - Pack count: 90s- Baddi - NDC:33342-074-10 - Valsartan and Hydrochlorothiazide Tablets, 80/12.5 mg - Pack count:90s-Daman ...
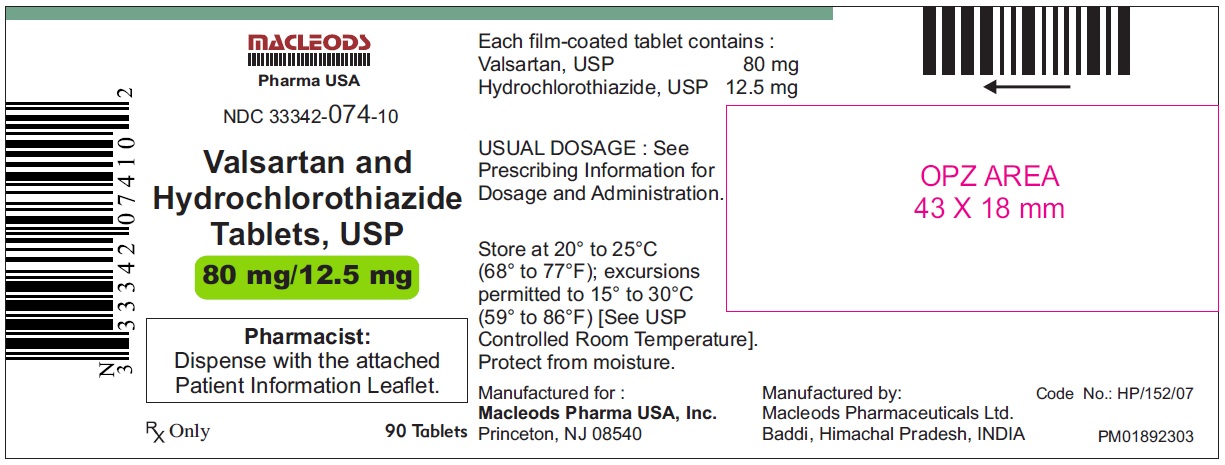
Valsartan and Hydrochlorothiazide Tablets, 80/12.5 mg
Pack count: 90s- Baddi
NDC:33342-074-10
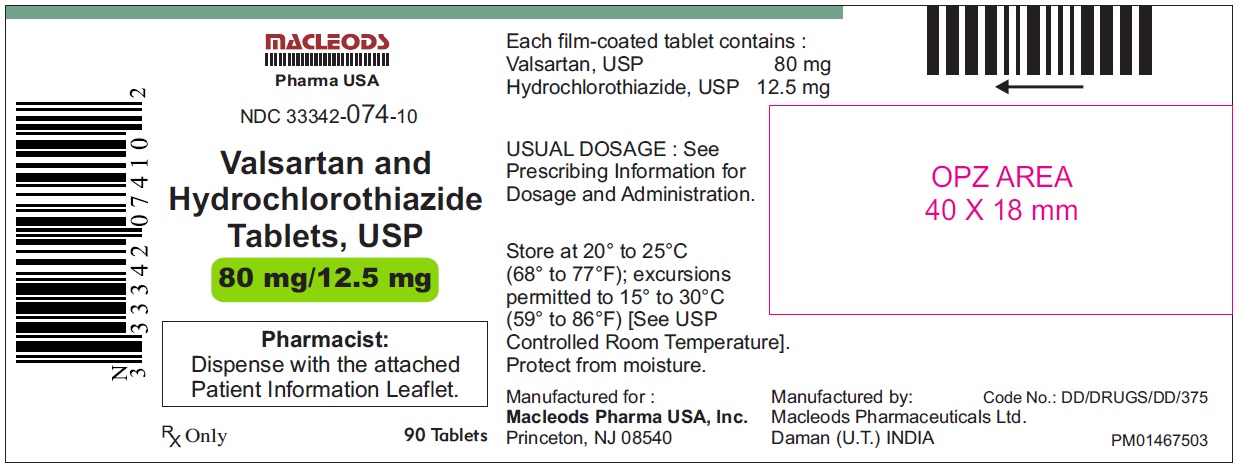
Valsartan and Hydrochlorothiazide Tablets, 80/12.5 mg
Pack count:90s-Daman
NDC:33342-074-10
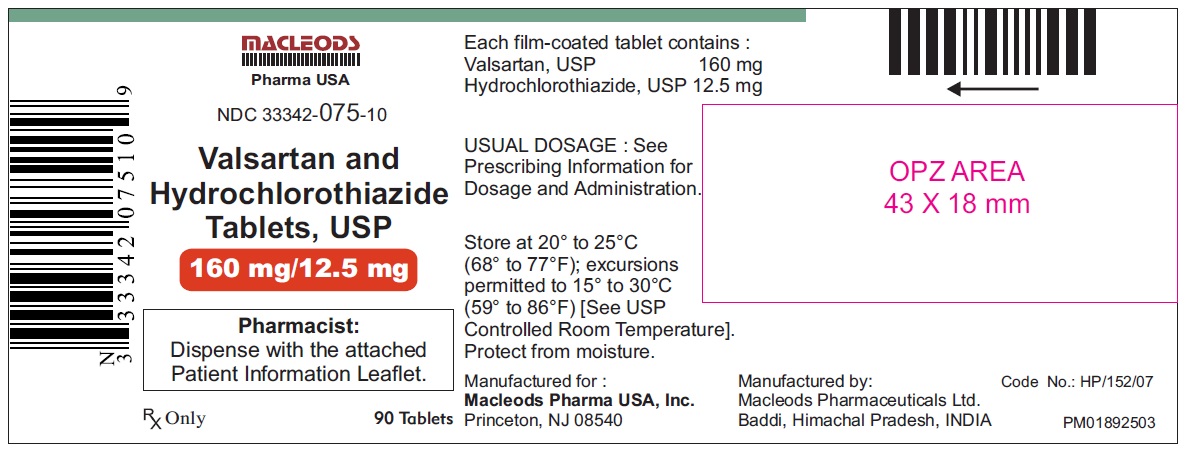
Valsartan and Hydrochlorothiazide Tablets, 160/12.5 mg
Pack count:90s-Baddi
NDC:33342-075-10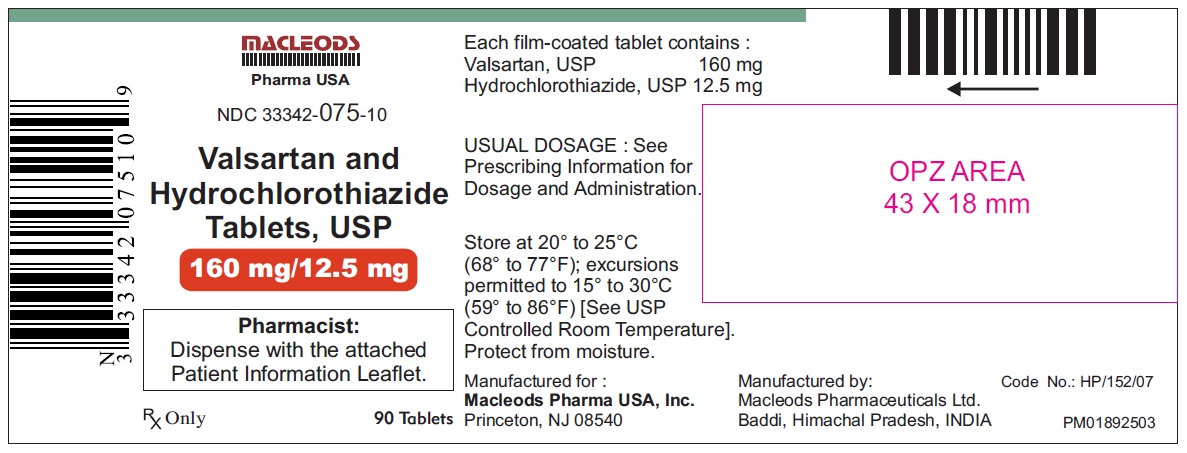
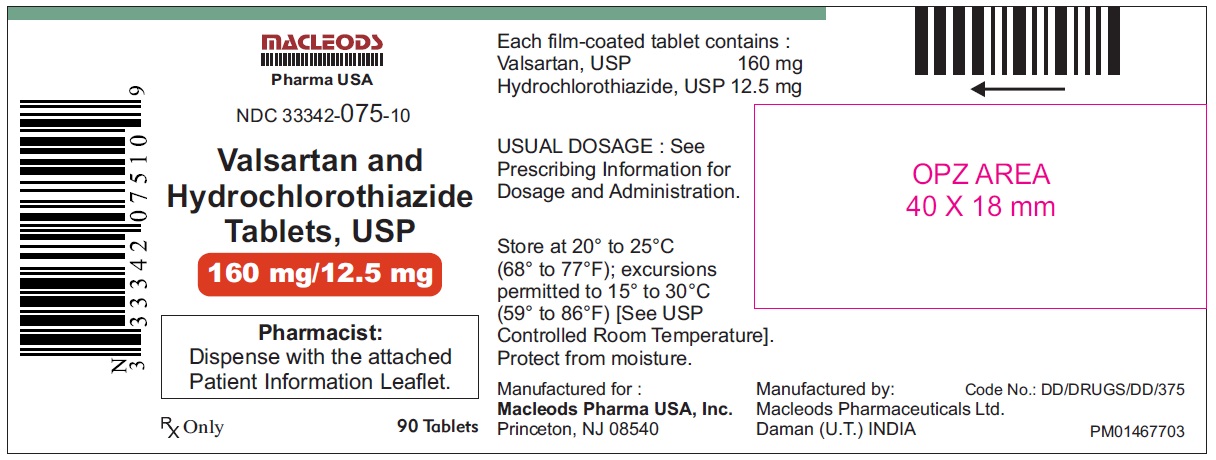
Valsartan and Hydrochlorothiazide Tablets, 160/12.5 mg
Pack count: 90s- Daman
NDC:33342-075-10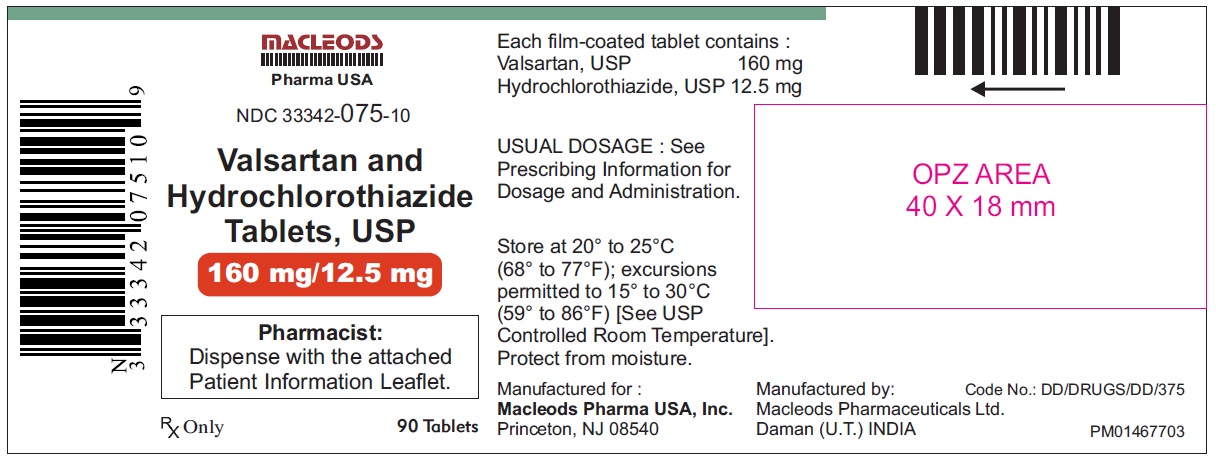
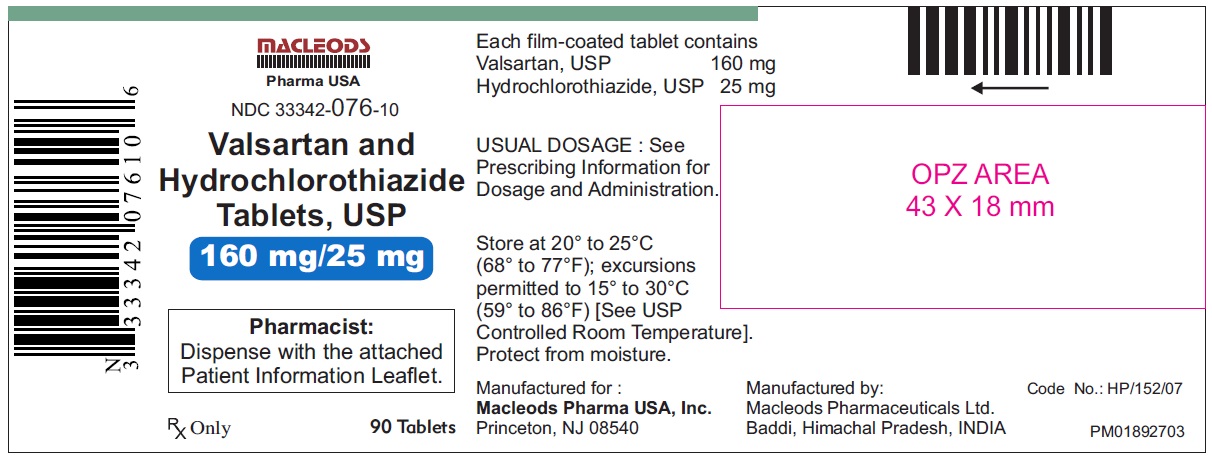
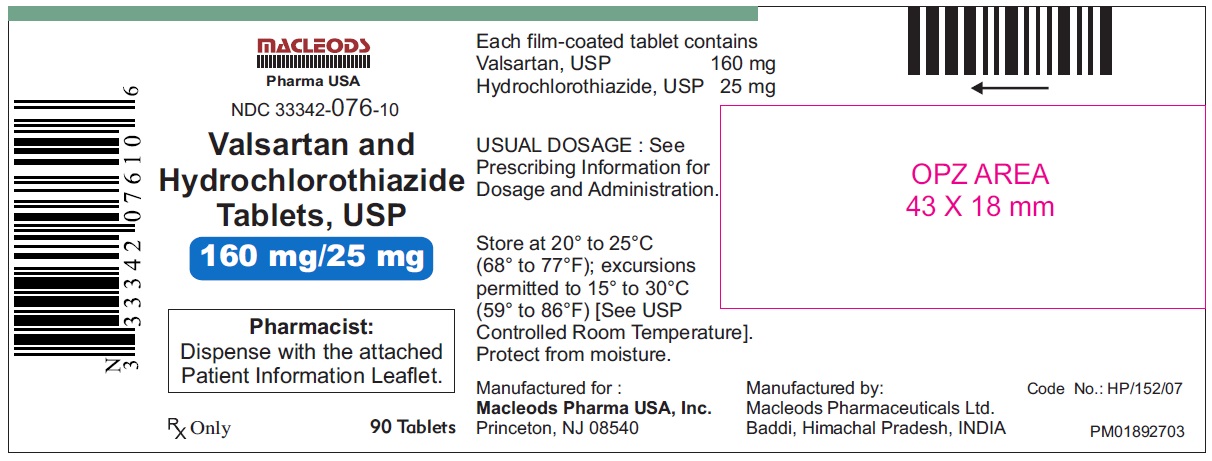
Valsartan and Hydrochlorothiazide Tablets, 160/25 mg
Pack count:90s-Baddi
NDC:33342-076-10
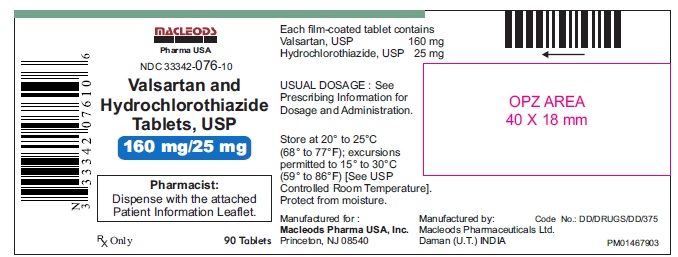
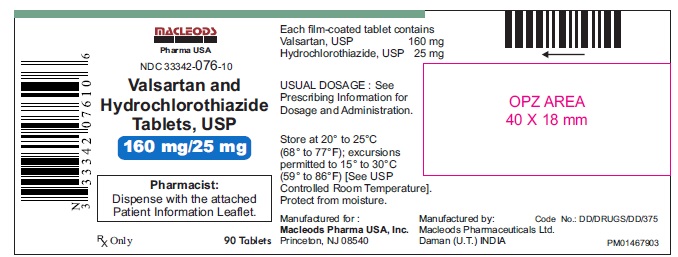
Valsartan and Hydrochlorothiazide Tablets,160/25 mg
Pack count:90s- Daman
NDC:33342-076-10
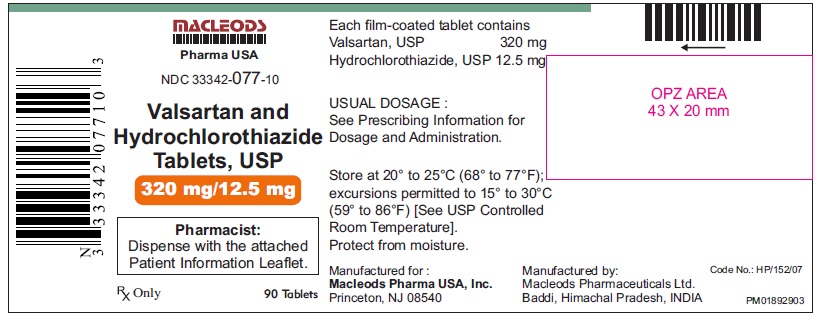
Valsartan and Hydrochlorothiazide Tablets, 320/12.5 mg
Pack count:90s- Baddi
NDC: 33342-077-10
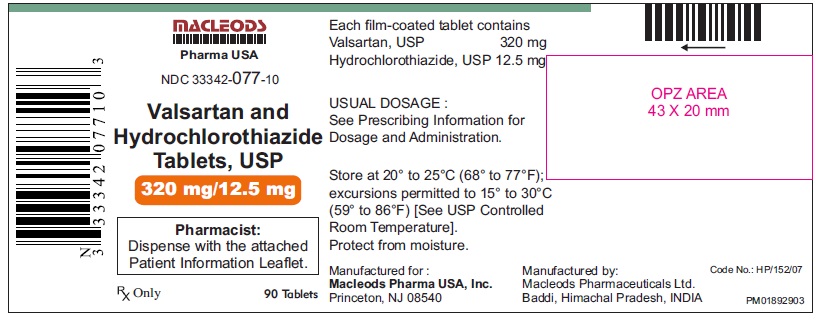
Valsartan and Hydrochlorothiazide Tablets, 320/12.5 mg
Pack count:90s-Daman
NDC:33342-077-10

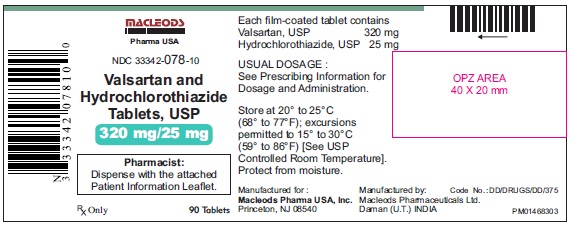
Valsartan and Hydrochlorothiazide Tablets, 320/25 mg
Pack count: 90s-Baddi
NDC: 33342-078-10

Valsartan and Hydrochlorothiazide Tablets, 320/25 mg
Pack count: 90s-Daman
NDC: 33342-078-10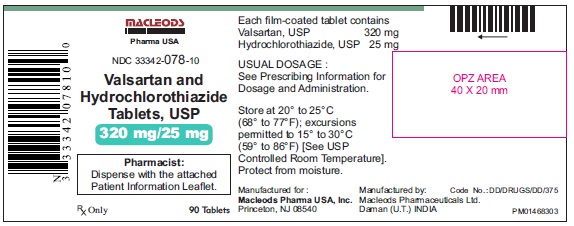
Close -
INGREDIENTS AND APPEARANCEProduct Information
VALSARTAN AND HYDROCHLOROTHIAZIDE valsartan and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-078 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 320 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color YELLOW Score no score Shape OVAL (Biconvex) Size 21mm Flavor Imprint Code L20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-078-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 2 NDC:33342-078-15 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203145 04/19/2013 VALSARTAN AND HYDROCHLOROTHIAZIDE valsartan and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-074 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 80 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color ORANGE (Light Orange) Score no score Shape OVAL (Biconvex) Size 13mm Flavor Imprint Code L16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-074-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 2 NDC:33342-074-44 1000 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203145 04/19/2013 VALSARTAN AND HYDROCHLOROTHIAZIDE valsartan and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-075 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 160 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color RED (Dark red) Score no score Shape OVAL (Biconvex) Size 17mm Flavor Imprint Code L17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-075-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 2 NDC:33342-075-15 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203145 04/19/2013 VALSARTAN AND HYDROCHLOROTHIAZIDE valsartan and hydrochlorothiazide tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-076 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 160 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color ORANGE (Brown Orange) Score no score Shape OVAL (Biconvex) Size 17mm Flavor Imprint Code L18 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-076-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 2 NDC:33342-076-15 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203145 04/19/2013 VALSARTAN AND HYDROCHLOROTHIAZIDE valsartan and hydrochlorothiazide tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-077 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 320 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color PINK Score no score Shape OVAL (Biconvex) Size 21mm Flavor Imprint Code L19 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-077-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 2 NDC:33342-077-15 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203145 04/19/2013 Labeler - Macleods Pharmaceuticals Limited (862128535) Establishment Name Address ID/FEI Business Operations Macleods Pharmaceuticals Limited 918608365 ANALYSIS(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , LABEL(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , MANUFACTURE(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , PACK(33342-074, 33342-075, 33342-076, 33342-077, 33342-078)
CloseEstablishment Name Address ID/FEI Business Operations Macleods Pharmaceuticals Limited 676369519 ANALYSIS(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , LABEL(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , MANUFACTURE(33342-074, 33342-075, 33342-076, 33342-077, 33342-078) , PACK(33342-074, 33342-075, 33342-076, 33342-077, 33342-078)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet
Number of versions: 8
RxNorm
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 200284 | HCTZ 12.5 MG / valsartan 80 MG Oral Tablet | PSN |
| 2 | 200284 | hydrochlorothiazide 12.5 MG / valsartan 80 MG Oral Tablet | SCD |
| 3 | 200284 | HCTZ 12.5 MG / valsartan 80 MG Oral Tablet | SY |
| 4 | 200285 | valsartan 160 MG / hydroCHLOROthiazide 12.5 MG Oral Tablet | PSN |
| 5 | 200285 | hydrochlorothiazide 12.5 MG / valsartan 160 MG Oral Tablet | SCD |
| 6 | 200285 | HCTZ 12.5 MG / valsartan 160 MG Oral Tablet | SY |
| 7 | 349353 | valsartan 160 MG / hydroCHLOROthiazide 25 MG Oral Tablet | PSN |
| 8 | 349353 | hydrochlorothiazide 25 MG / valsartan 160 MG Oral Tablet | SCD |
| 9 | 349353 | HCTZ 25 MG / valsartan 160 MG Oral Tablet | SY |
| 10 | 636042 | valsartan 320 MG / hydroCHLOROthiazide 12.5 MG Oral Tablet | PSN |
| 11 | 636042 | hydrochlorothiazide 12.5 MG / valsartan 320 MG Oral Tablet | SCD |
| 12 | 636042 | HCTZ 12.5 MG / valsartan 320 MG Oral Tablet | SY |
| 13 | 636045 | valsartan 320 MG / hydroCHLOROthiazide 25 MG Oral Tablet | PSN |
| 14 | 636045 | hydrochlorothiazide 25 MG / valsartan 320 MG Oral Tablet | SCD |
| 15 | 636045 | HCTZ 25 MG / valsartan 320 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=771afba6-8769-4ea8-afec-358dba0aaefe
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
VALSARTAN AND HYDROCHLOROTHIAZIDE tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 33342-074-10 |
| 2 | 33342-074-44 |
| 3 | 33342-075-10 |
| 4 | 33342-075-15 |
| 5 | 33342-076-10 |
| 6 | 33342-076-15 |
| 7 | 33342-077-10 |
| 8 | 33342-077-15 |
| 9 | 33342-078-10 |
| 10 | 33342-078-15 |