The clinical efficacy studies described below were conducted with memantine hydrochloride tablets and not with memantine hydrochloride oral solution; however, bioequivalence of memantine ...
The clinical efficacy studies described below were conducted with memantine hydrochloride tablets and not with memantine hydrochloride oral solution; however, bioequivalence of memantine hydrochloride oral solution with memantine hydrochloride tablets has been demonstrated.
The effectiveness of memantine hydrochloride as a treatment for patients with moderate to severe Alzheimer's disease was demonstrated in 2 randomized, double-blind, placebo-controlled clinical studies (Studies 1 and 2) conducted in the United States that assessed both cognitive function and day to day function. The mean age of patients participating in these two trials was 76 with a range of 50 to 93 years. Approximately 66% of patients were female and 91% of patients were Caucasian. A third study (Study 3), carried out in Latvia, enrolled patients with severe dementia, but did not assess cognitive function as a planned endpoint. Study Outcome Measures: In each U.S. study, the effectiveness of memantine hydrochloride was determined using both an instrument designed to evaluate overall function through caregiver-related assessment, and an instrument that measures cognition. Both studies showed that patients on memantine hydrochloride experienced significant improvement on both measures compared to placebo.
Day-to-day function was assessed in both studies using the modified Alzheimer's disease Cooperative Study -Activities of Daily Living inventory (ADCS-ADL). The ADCS-ADL consists of a comprehensive battery of ADL questions used to measure the functional capabilities of patients. Each ADL item is rated from the highest level of independent performance to complete loss. The investigator performs the inventory by interviewing a caregiver familiar with the behavior of the patient. A subset of 19 items, including ratings of the patient's ability to eat, dress, bathe, telephone, travel, shop, and perform other household chores has been validated for the assessment of patients with moderate to severe dementia. This is the modified ADCS-ADL, which has a scoring range of 0 to 54, with the lower scores indicating greater functional impairment.
The ability of memantine hydrochloride to improve cognitive performance was assessed in both studies with the Severe Impairment Battery (SIB), a multi-item instrument that has been validated for the evaluation of cognitive function in patients with moderate to severe dementia. The SIB examines selected aspects of cognitive performance, including elements of attention, orientation, language, memory, visuospatial ability, construction, praxis, and social interaction. The SIB scoring range is from 0 to 100, with lower scores indicating greater cognitive impairment.
Study 1 (Twenty-Eight-Week Study)
In a study of 28 weeks duration, 252 patients with moderate to severe probable Alzheimer's disease (diagnosed by DSM-IV and NINCDS-ADRDA criteria, with Mini-Mental State Examination scores ≥ 3 and ≤ 14 and Global Deterioration Scale Stages 5-6) were randomized to memantine hydrochloride or placebo. For patients randomized to memantine hydrochloride, treatment was initiated at 5 mg once daily and increased weekly by 5 mg/day in divided doses to a dose of 20 mg/day (10 mg twice a day).
Effects on the ADCS-ADL
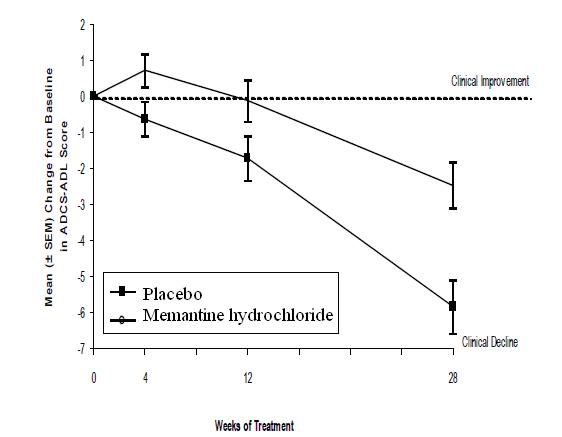
Figure 1 shows the time course for the change from baseline in the ADCS-ADL score for patients in the two treatment groups completing the 28 weeks of the study. At 28 weeks of treatment, the mean difference in the ADCS-ADL change scores for the memantine hydrochloride -treated patients compared to the patients on placebo was 3.4 units. Using an analysis based on all patients and carrying their last study observation forward (LOCF analysis), memantine hydrochloride treatment was statistically significantly superior to placebo.
Figure 1: Time course of the change from baseline in ADCS-ADL score for patients completing 28 weeks of treatment
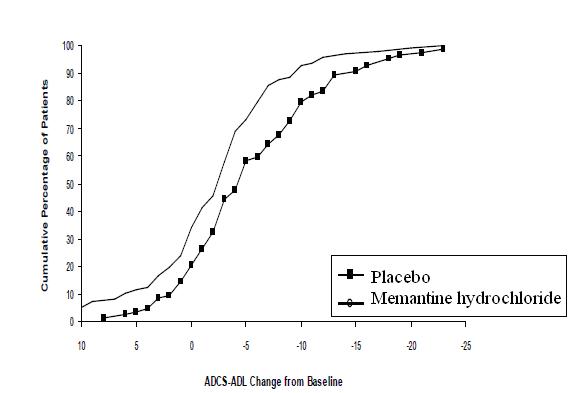
Figure 2 shows the cumulative percentages of patients from each of the treatment groups who had attained at least the change in the ADCS-ADL shown on the X axis. The curves show that both patients assigned to memantine hydrochloride and placebo have a wide range of responses and generally show deterioration (a negative change in ADCS-ADL compared to baseline), but that the memantine hydrochloride group is more likely to show a smaller decline or an improvement. (In a cumulative distribution display, a curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon or shifted to the right of the curve for placebo).
Figure 2: Cumulative percentage of patients completing 28 weeks of double-blind treatment with specified changes from baseline in ADCS-ADL scores.
Effects on the SIB
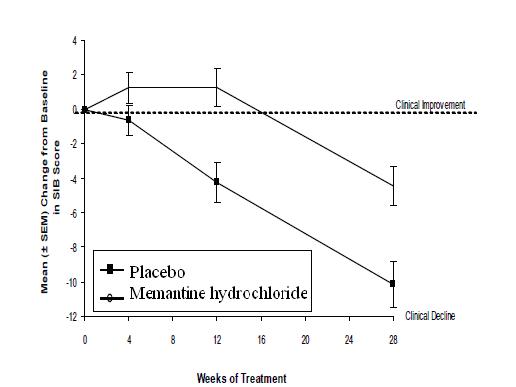
Figure 3 shows the time course for the change from baseline in SIB score for the two treatment groups over the 28 weeks of the study. At 28 weeks of treatment, the mean difference in the SIB change scores for the memantine hydrochloride -treated patients compared to the patients on placebo was 5.7 units. Using an LOCF analysis, memantine hydrochloride treatment was statistically significantly superior to placebo.
Figure 3: Time course of the change from baseline in SIB score for patients completing 28 weeks of treatment
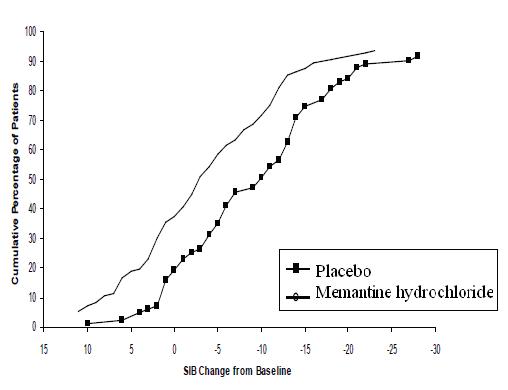
Figure 4 shows the cumulative percentages of patients from each treatment group who had attained at least the measure of change in SIB score shown on the X axis. The curves show that both patients assigned to memantine hydrochloride and placebo have a wide range of responses and generally show deterioration, but that the memantine hydrochloride group is more likely to show a smaller decline or an improvement.
Figure 4: Cumulative percentage of patients completing 28 weeks of double-blind treatment with specified changes from baseline in SIB scores.
Study 2 (Twenty-Four-Week Study)
In a study of 24 weeks duration, 404 patients with moderate to severe probable Alzheimer's disease (diagnosed by NINCDS-ADRDA criteria, with Mini-Mental State Examination scores ≥ 5 and ≤ 14) who had been treated with donepezil for at least 6 months and who had been on a stable dose of donepezil for the last 3 months were randomized to memantine hydrochloride or placebo while still receiving donepezil. For patients randomized to memantine hydrochloride, treatment was initiated at 5 mg once daily and increased weekly by 5 mg/day in divided doses to a dose of 20 mg/day (10 mg twice a day).
Effects on the ADCS-ADL
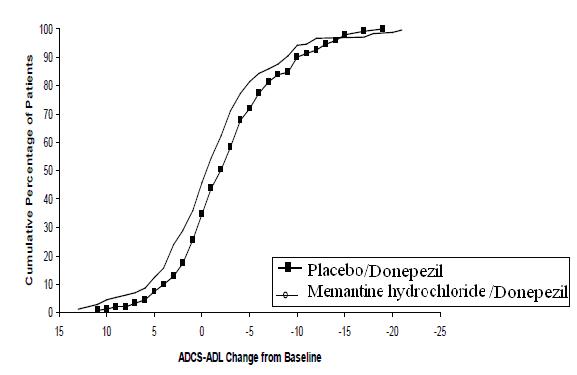
Figure 5 shows the time course for the change from baseline in the ADCS-ADL score for the two treatment groups over the 24 weeks of the study. At 24 weeks of treatment, the mean difference in the ADCS-ADL change scores for the memantine hydrochloride /donepezil treated patients (combination therapy) compared to the patients on placebo/donepezil (monotherapy) was 1.6 units. Using an LOCF analysis, memantine hydrochloride /donepezil treatment was statistically significantly superior to placebo/donepezil.
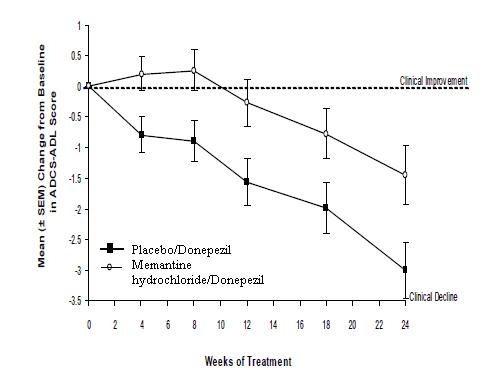
Figure 5: Time course of the change from baseline in ADCS-ADL score for patients completing 24 weeks of treatment.
Figure 6 shows the cumulative percentages of patients from each of the treatment groups who had attained at least the measure of improvement in the ADCS-ADL shown on the X axis. The curves show that both patients assigned to memantine hydrochloride/donepezil and placebo/donepezil have a wide range of responses and generally show deterioration, but that the memantine hydrochloride /donepezil group is more likely to show a smaller decline or an improvement.
Figure 6: Cumulative percentage of patients completing 24 weeks of double-blind treatment with specified changes from baseline in ADCS-ADL scores.
Effects on the SIB
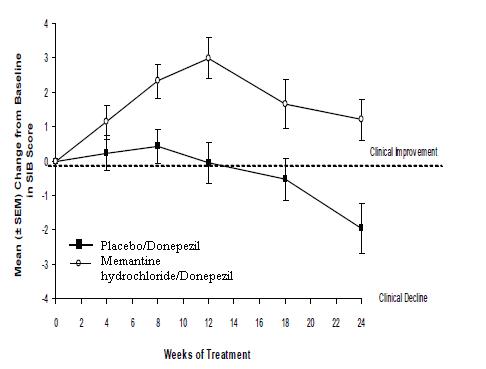
Figure 7 shows the time course for the change from baseline in SIB score for the two treatment groups over the 24 weeks of the study. At 24 weeks of treatment, the mean difference in the SIB change scores for the memantine hydrochloride /donepezil-treated patients compared to the patients on placebo/donepezil was 3.3 units. Using an LOCF analysis, memantine hydrochloride /donepezil treatment was statistically significantly superior to placebo/donepezil.
Figure 7: Time course of the change from baseline in SIB score for patients completing 24 weeks of treatment.
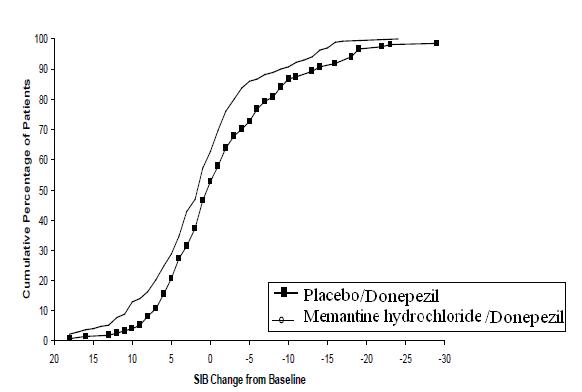
Figure 8 shows the cumulative percentages of patients from each treatment group who had attained at least the measure of improvement in SIB score shown on the X axis. The curves show that both patients assigned to memantine hydrochloride/donepezil and placebo/donepezil have a wide range of responses, but that the memantine hydrochloride /donepezil group is more likely to show an improvement or a smaller decline.
Figure 8: Cumulative percentage of patients completing 24 weeks of double-blind treatment with specified changes from baseline in SIB scores.
Study 3 (Twelve-Week Study)
In a double-blind study of 12 weeks duration, conducted in nursing homes in Latvia, 166 patients with dementia according to DSM-III-R, a Mini-Mental State Examination score of < 10, and Global Deterioration Scale staging of 5 to 7 were randomized to either memantine hydrochloride or placebo. For patients randomized to memantine hydrochloride, treatment was initiated at 5 mg once daily and increased to 10 mg once daily after 1 week. The primary efficacy measures were the care dependency subscale of the Behavioral Rating Scale for Geriatric Patients (BGP), a measure of day-to-day function, and a Clinical Global Impression of Change (CGI-C), a measure of overall clinical effect. No valid measure of cognitive function was used in this study. A statistically significant treatment difference at 12 weeks that favored memantine hydrochloride over placebo was seen on both primary efficacy measures. Because the patients entered were a mixture of Alzheimer's disease and vascular dementia, an attempt was made to distinguish the two groups and all patients were later designated as having either vascular dementia or Alzheimer's disease, based on their scores on the Hachinski Ischemic Scale at study entry. Only about 50% of the patients had computerized tomography of the brain. For the subset designated as having Alzheimer's disease, a statistically significant treatment effect favoring memantine hydrochloride over placebo at 12 weeks was seen on both the BGP and CGI-C.
Close