Label: LAMIVUDINE AND ZIDOVUDINE tablet
- NDC Code(s): 33342-003-09
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LAMIVUDINE and ZIDOVUDINE TABLETS safely and effectively. See full prescribing information for LAMIVUDINE and ZIDOVUDINE TABLETS ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEMATOLOGIC TOXICITY, MYOPATHY, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, and EXACERBATIONS OF HEPATITIS B
Zidovudine, a component of lamivudine and zidovudine tablets, has been associated with hematologic toxicity including neutropenia and severe anemia, particularly in patients with advanced Human Immunodeficiency Virus (HIV-1) disease [see Warnings and Precautions (5.1)].
Close
Prolonged use of zidovudine has been associated with symptomatic myopathy [see Warnings and Precautions (5.2)].
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including lamivudine and zidovudine (components of lamivudine and zidovudine tablets). Discontinue lamivudine and zidovudine tablets if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur [see Warnings and Precautions (5.3)].
Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and HIV-1 and have discontinued lamivudine, which is one component of lamivudine and zidovudine tablets. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue lamivudine and zidovudine tablets and are co-infected with HIV-1 and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.4)]. -
1 INDICATIONS & USAGELamivudine and zidovudine tablets, a combination of 2 nucleoside analogues, is indicated in combination with other antiretrovirals for the treatment of human immunodeficiency virus type 1 (HIV-1 ...
-
2 DOSAGE & ADMINISTRATION2.1 Recommended Dosage for Adults and Adolescents - The recommended dosage of lamivudine and zidovudine tablets in HIV-1-infected adults and adolescents weighing greater than or equal to 30 kg is ...
-
3 DOSAGE FORMS & STRENGTHSLamivudine and zidovudine tablets, USP contain 150 mg of lamivudine and 300 mg of zidovudine. The tablets are white coloured, biconvex, modified capsule shaped, film-coated tablet having score on ...
-
4 CONTRAINDICATIONSLamivudine and zidovudine tablets are contraindicated in patients with a previous hypersensitivity reaction to lamivudine or zidovudine.
-
5 WARNINGS AND PRECAUTIONS5.1 Hematologic Toxicity/Bone Marrow Suppression - Zidovudine, a component of lamivudine and zidovudine tablets, has been associated with hematologic toxicity including neutropenia and anemia ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: • Hematologic toxicity, including neutropenia and anemia [see Boxed Warning, Warnings and Precautions (5.1)] ...
-
7 DRUG INTERACTIONS7.1 Zidovudine - Agents Antagonistic with Zidovudine - Concomitant use of zidovudine with the following drugs should be avoided since an antagonistic relationship has been demonstrated in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lamivudine and zidovudine tablets during pregnancy ...
-
10 OVERDOSAGEThere is no known specific treatment for overdose with lamivudine and zidovudine tablets. If overdose occurs, the patient should be monitored and standard supportive treatment applied as ...
-
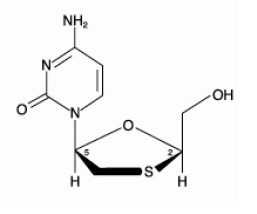
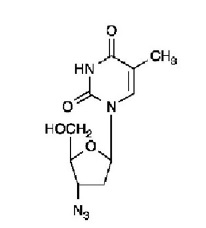
11 DESCRIPTIONLamivudine and zidovudine tablets, USP are combination tablets containing lamivudine and zidovudine. Lamivudine and zidovudine (azidothymidine, AZT, or ZDV) are synthetic nucleoside analogues with ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lamivudine and zidovudine is an antiretroviral agent [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Pharmacokinetics in Adults - One lamivudine and zidovudine ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Carcinogenicity - Lamivudine: Long-term carcinogenicity studies with lamivudine in mice and rats showed no evidence of carcinogenic ...
-
14 CLINICAL STUDIESOne lamivudine and zidovudine tablet given twice daily is an alternative regimen to EPIVIR tablets 150 mg twice daily plus RETROVIR 600 mg per day in divided doses. 14.1 Adults - The NUCB3007 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLamivudine and Zidovudine Tablets, USP containing 150 mg lamivudine and 300 mg zidovudine, are white, film-coated, modified capsule shaped tablets with score on one side and 'ML 6' debossed on the ...
-
17 PATIENT COUNSELING INFORMATIONNeutropenia and Anemia - Inform patients that the important toxicities associated with zidovudine are neutropenia and/or anemia. Inform them of the extreme importance of having their blood counts ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELLamivudine and Zidovudine Tablets, USP 150 mg/300 mg - 60's Container Pack - NDC : 33342-003-09 - Lamivudine and Zidovudine Tablets, USP 150 mg/300 mg - 6 x 10 unit dose - NDC : 33342-003-24 ...
-
INGREDIENTS AND APPEARANCEProduct Information