Label: LEVOCETIRIZINE DIHYDROCHLORIDE solution
- NDC Code(s): 31722-659-31
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOCETIRIZINE DIHYDROCHLORIDE ORAL SOLUTION safely and effectively. See full prescribing information for LEVOCETIRIZINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.1 Perennial Allergic Rhinitis - Levocetirizine dihydrochloride oral solution is indicated for the relief of symptoms associated with perennial allergic rhinitis in children 6 months to 2 years ...
-
2 DOSAGE & ADMINISTRATIONLevocetirizine dihydrochloride is available as 2.5 mg/5 mL (0.5 mg/mL) oral solution. Levocetirizine dihydrochloride oral solution can be taken without regard to food consumption. 2.1 ...
-
3 DOSAGE FORMS & STRENGTHSLevocetirizine dihydrochloride oral solution is a clear, colorless liquid containing 0.5 mg of levocetirizine dihydrochloride per mL.
-
4 CONTRAINDICATIONSThe use of levocetirizine dihydrochloride oral solution is contraindicated in: 4.1 Patients with Known Hypersensitivity - Patients with known hypersensitivity to levocetirizine or any of the ...
-
5 WARNINGS AND PRECAUTIONS5.1 Somnolence - In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with levocetirizine dihydrochloride. Patients should be ...
-
6 ADVERSE REACTIONSUse of levocetirizine dihydrochloride oral solution has been associated with somnolence, fatigue, asthenia, and urinary retention [see Warnings and Precautions (5)]. 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONSIn vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience with levocetirizine use in pregnant women are insufficient to identify any drug-associated ...
-
10 OVERDOSAGEOverdosage has been reported with levocetirizine dihydrochloride. Symptoms of overdose may include drowsiness in adults. In children agitation and restlessness may initially occur, followed ...
-
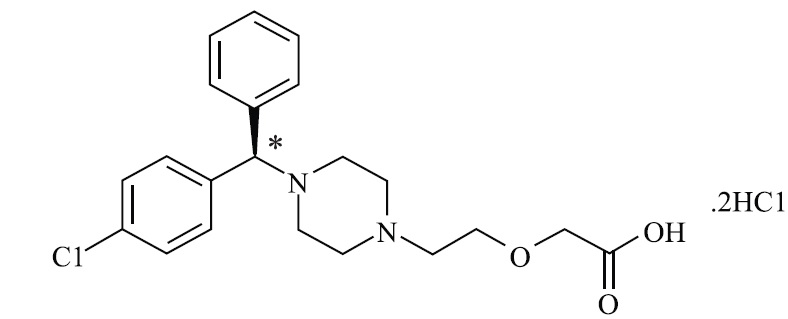
11 DESCRIPTIONLevocetirizine dihydrochloride, the active component of levocetirizine dihydrochloride oral solution is an orally active H1-receptor antagonist. The chemical name is 2-[2-[4[(R)-(4-chlorophenyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Levocetirizine, the active enantiomer of cetirizine, is an antihistamine; its principal effects are mediated via selective inhibition of H1 receptors. The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - No carcinogenicity studies have been performed with levocetirizine. However, evaluation of cetirizine carcinogenicity studies is ...
-
14 CLINICAL STUDIES14.1 Perennial Allergic Rhinitis - Adults and Adolescents 12 Years of Age and Older - The efficacy of levocetirizine dihydrochloride was evaluated in four randomized, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLevocetirizine dihydrochloride oral solution is a clear, colorless liquid containing 0.5 mg of levocetirizine dihydrochloride per mL. Oral Solution available in 5 oz (148 mL) polypropylene ...
-
17 PATIENT COUNSELING INFORMATIONSomnolence - Caution patients against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELLevocetirizine Dihydrochloride Oral Solution 2.5 mg/ 5 mL (0.5 mg/mL) container label - Levocetirizine Dihydrochloride Oral Solution 2.5 mg/ 5 mL (0.5 mg/mL) carton label
-
INGREDIENTS AND APPEARANCEProduct Information