Label: ICOSAPENT ETHYL capsule
- NDC Code(s): 31722-298-24, 31722-299-12
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ICOSAPENT ETHYL CAPSULES safely and effectively. See full prescribing information for ICOSAPENT ETHYL CAPSULES. ICOSAPENT ETHYL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEIcosapent ethyl capsules are indicated: as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. Limitations of Use: The ...
-
2 DOSAGE AND ADMINISTRATION2.1 Prior to Initiation of Icosapent Ethyl Capsules - Assess lipid levels before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, or medications) of high ...
-
3 DOSAGE FORMS AND STRENGTHSIcosapent ethyl capsules are supplied as: 0.5 gram clear, transparent oval capsules containing clear to light yellow colored solution imprinted with ‘547’ with white ink. 1 gram clear ...
-
4 CONTRAINDICATIONSIcosapent ethyl capsules are contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to icosapent ethyl or any of its components.
-
5 WARNINGS AND PRECAUTIONS5.1 Atrial Fibrillation/Flutter - Icosapent ethyl is associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization. In a double-blind, placebo-controlled ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described below and elsewhere in the labeling: Atrial Fibrillation or Atrial Flutter [see Warnings and Precautions (5.1)] Potential for Allergic ...
-
7 DRUG INTERACTIONS7.1 Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents - Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. The prolongation of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data from published case reports and the pharmacovigilance database on the use of icosapent ethyl in pregnant women are insufficient to identify a ...
-
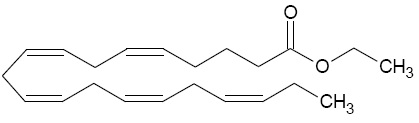
11 DESCRIPTIONIcosapent ethyl capsules, a lipid-regulating agent, is supplied as either a 0.5 gram or a 1 gram liquid-filled clear, transparent soft gelatin capsules containing clear to light yellow colored ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Studies suggest that EPA reduces hepatic very low-density lipoprotein triglycerides (VLDL-TG) synthesis and/or secretion and enhances TG clearance from circulating VLDL ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year rat carcinogenicity study with oral gavage doses of 0.09, 0.27, and 0.91 g/kg/day icosapent ethyl, respectively, males did ...
-
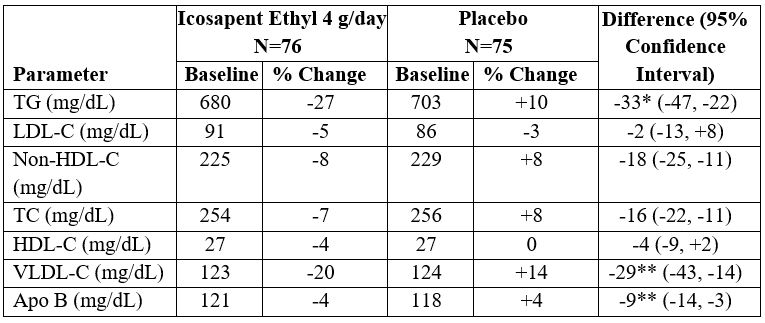
14 CLINICAL STUDIES14.2 Severe Hypertriglyceridemia - The effects of icosapent ethyl 4 grams per day were assessed in a randomized, placebo-controlled, double-blind, parallel-group study of adult patients (76 on ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIcosapent ethyl capsules are supplied as: The 0.5 gram icosapent ethyl capsule is available as a clear, transparent oval capsules containing clear to light yellow colored solution imprinted with ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling before starting icosapent ethyl capsules (Patient Information). Inform patients that icosapent ethyl capsules may increase their risk ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Icosapent Ethyl (eye koe’ sa pent eth il) Capsules - What are Icosapent ethyl capsules? Icosapent ethyl capsules are a prescription medicine used: along with a ...
-
PRINCIPAL DISPLAY PANELIcosapent ethyl capsules 0.5 gram - 240 Counts Label - Icosapent ethyl capsules 1 gram - 120 Counts Label
-
INGREDIENTS AND APPEARANCEProduct Information