Label: LEVOTHYROXINE SODIUM tablet
-
NDC Code(s):
31722-284-01,
31722-284-10,
31722-284-30,
31722-284-90, view more31722-285-01, 31722-285-10, 31722-285-30, 31722-285-90, 31722-286-01, 31722-286-10, 31722-286-30, 31722-286-90, 31722-287-01, 31722-287-10, 31722-287-30, 31722-287-90, 31722-288-01, 31722-288-10, 31722-288-30, 31722-288-90, 31722-289-01, 31722-289-10, 31722-289-30, 31722-289-90, 31722-290-01, 31722-290-10, 31722-290-30, 31722-290-90, 31722-291-01, 31722-291-10, 31722-291-30, 31722-291-90, 31722-292-01, 31722-292-10, 31722-292-30, 31722-292-90, 31722-293-01, 31722-293-10, 31722-293-30, 31722-293-90, 31722-294-01, 31722-294-10, 31722-294-30, 31722-294-90, 31722-295-01, 31722-295-10, 31722-295-30, 31722-295-90
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOTHYROXINE SODIUM TABLETS safely and effectively. See full prescribing information for LEVOTHYROXINE SODIUM ...These highlights do not include all the information needed to use LEVOTHYROXINE SODIUM TABLETS safely and effectively.
See full prescribing information for LEVOTHYROXINE SODIUM TABLETS.
LEVOTHYROXINE SODIUM tablets, for oral use
Initial U.S. Approval: 2002WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
See full prescribing information for complete boxed warning
- Thyroid hormones, including levothyroxine sodium tablets should not be used for the treatment of obesity or for weight loss.
- Doses beyond the range of daily hormonal requirements may produce serious or even life threatening manifestations of toxicity (6, 10).
INDICATIONS AND USAGE
Levothyroxine sodium tablets are L-thyroxine (T4) indicated for: (1)
- Hypothyroidism: As replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism. (1)
- Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) Suppression: As an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer. (1)
Limitations of Use: (1)
- Not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients. (1)
- Not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis. (1)
DOSAGE AND ADMINISTRATION
- Administer once daily, preferably on an empty stomach, one-half to one hour before breakfast. (2.1)
- Administer at least 4 hours before or after drugs that are known to interfere with absorption. (2.1)
- Evaluate the need for dose adjustments when regularly administering within one hour of certain foods that may affect absorption. (2.1)
- Starting dose depends on a variety of factors, including age, body weight, cardiovascular status, and concomitant medications. Peak therapeutic effect may not be attained for 4-6 weeks. (2.2)
- See full prescribing information for dosing in specific patient populations. (2.3)
- Adequacy of therapy determined with periodic monitoring of TSH and/or T4 as well as clinical status. (2.4)
DOSAGE FORMS AND STRENGTHS
Tablets: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg, and 300 mcg (3) (3)
CONTRAINDICATIONS
- Uncorrected adrenal insufficiency. (4)
WARNINGS AND PRECAUTIONS
- Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate levothyroxine sodium tablets at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation. (2.3, 5.1, 8.5)
- Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.2)
- Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of levothyroxine sodium tablets treatment. (5.3)
- Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism. (5.4)
- Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy. (5.5)
- Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose. (5.6)
ADVERSE REACTIONS
Adverse reactions associated with levothyroxine sodium tablets therapy are primarily those of hyperthyroidism due to therapeutic overdosage: arrhythmias, myocardial infarction, dyspnea, muscle spasm, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Camber Pharmaceuticals, Inc., at 1-866-495-8330 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to levothyroxine sodium tablets. (7)
USE IN SPECIFIC POPULATIONS
Pregnancy may require the use of higher doses of levothyroxine sodium tablets. (2.3, 8.1) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Information
2.2 General Principles of Dosing
2.3 Dosing in Specific Patient Populations
2.4 Monitoring TSH and/or Thyroxine (T4) Levels
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
5.2 Myxedema Coma
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
5.5 Worsening of Diabetic Control
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-Replacement
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
7.2 Antidiabetic Therapy
7.3 Oral Anticoagulants
7.4 Digitalis Glycosides
7.5 Antidepressant Therapy
7.6 Ketamine
7.7 Sympathomimetics
7.8 Tyrosine-Kinase Inhibitors
7.9 Drug-Food Interactions
7.10 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
Thyroid hormones, including levothyroxine sodium tablets, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), and Overdosage (10)].
Close -
1 INDICATIONS AND USAGEHypothyroidism - Levothyroxine sodium tablets are indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired ...
Hypothyroidism
Levothyroxine sodium tablets are indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) Suppression
Levothyroxine sodium tablets are indicated as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer.
Limitations of Use:
- Levothyroxine sodium tablets are not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients as there are no clinical benefits and overtreatment with levothyroxine sodium tablets may induce hyperthyroidism [see Warnings and Precautions (5.4)].
- Levothyroxine sodium tablets are not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis.
-
2 DOSAGE AND ADMINISTRATION2.1 General Administration Information - Administer levothyroxine sodium tablets as a single daily dose, on an empty stomach, one-half to one hour before breakfast. Administer levothyroxine ...
2.1 General Administration Information
Administer levothyroxine sodium tablets as a single daily dose, on an empty stomach, one-half to one hour before breakfast.
Administer levothyroxine sodium tablets at least 4 hours before or after drugs known to interfere with levothyroxine sodium tablets absorption [see Drug Interactions (7.1)].
Evaluate the need for dose adjustments when regularly administering within one hour of certain foods that may affect levothyroxine sodium tablets absorption [see Drug Interactions (7.9) and Clinical Pharmacology (12.3)].
Administer levothyroxine sodium tablets to infants and children who cannot swallow intact tablets by crushing the tablet, suspending the freshly crushed tablet in a small amount (5 mL to 10 mL or 1 teaspoon to 2 teaspoons) of water and immediately administering the suspension by spoon or dropper. Do not store the suspension. Do not administer in foods that decrease absorption of levothyroxine sodium tablets, such as soybean-based infant formula [see Drug Interactions (7.9)].
2.2 General Principles of Dosing
The dose of levothyroxine sodium tablets for hypothyroidism or pituitary TSH suppression depends on a variety of factors including: the patient's age, body weight, cardiovascular status, concomitant medical conditions (including pregnancy), concomitant medications, co-administered food and the specific nature of the condition being treated [see Dosage and Administration (2.3), Warnings and Precautions (5), and Drug Interactions (7)]. Dosing must be individualized to account for these factors and dose adjustments made based on periodic assessment of the patient's clinical response and laboratory parameters [see Dosage and Administration (2.4)].
The peak therapeutic effect of a given dose of levothyroxine sodium tablets may not be attained for 4 to 6 weeks.
2.3 Dosing in Specific Patient Populations
Primary Hypothyroidism in Adults and in Adolescents in Whom Growth and Puberty are Complete
Start levothyroxine sodium tablets at the full replacement dose in otherwise healthy, non-elderly individuals who have been hypothyroid for only a short time (such as a few months). The average full replacement dose of levothyroxine sodium tablets is approximately 1.6 mcg per kg per day (for example: 100 mcg per day to 125 mcg per day for a 70 kg adult).
Adjust the dose by 12.5 mcg to 25 mcg increments every 4 to 6 weeks until the patient is clinically euthyroid and the serum TSH returns to normal. Doses greater than 200 mcg per day are seldom required. An inadequate response to daily doses of greater than 300 mcg per day is rare and may indicate poor compliance, malabsorption, drug interactions, or a combination of these factors.
For elderly patients or patients with underlying cardiac disease, start with a dose of 12.5 mcg per day to 25 mcg per day. Increase the dose every 6 to 8 weeks, as needed until the patient is clinically euthyroid and the serum TSH returns to normal. The full replacement dose of levothyroxine sodium tablets may be less than 1 mcg per kg per day in elderly patients.
In patients with severe longstanding hypothyroidism, start with a dose of 12.5 mcg per day to 25 mcg per day. Adjust the dose in 12.5 mcg to 25 mcg increments every 2 to 4 weeks until the patient is clinically euthyroid and the serum TSH level is normalized.
Secondary or Tertiary Hypothyroidism
Start levothyroxine sodium tablets at the full replacement dose in otherwise healthy, non-elderly individuals. Start with a lower dose in elderly patients, patients with underlying cardiovascular disease or patients with severe longstanding hypothyroidism as described above. Serum TSH is not a reliable measure of levothyroxine sodium tablets dose adequacy in patients with secondary or tertiary hypothyroidism and should not be used to monitor therapy. Use the serum free-T4 (L-thyroxine) level to monitor adequacy of therapy in this patient population. Titrate levothyroxine sodium tablets dosing per above instructions until the patient is clinically euthyroid and the serum free-T4 level is restored to the upper half of the normal range.
Pediatric Dosage -Congenital or Acquired Hypothyroidism
The recommended daily dose of levothyroxine sodium tablets in pediatric patients with hypothyroidism is based on body weight and changes with age as described in Table 1. Start levothyroxine sodium tablets at the full daily dose in most pediatric patients. Start at a lower starting dose in newborns (0-3 months) at risk for cardiac failure and in children at risk for hyperactivity (see below). Monitor for clinical and laboratory response [see Dosage and Administration (2.4)].
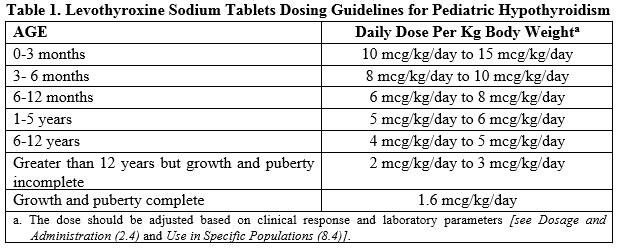
Newborns (0-3 months) at risk for cardiac failure: Consider a lower starting dose in newborns at risk for cardiac failure. Increase the dose every 4 to 6 weeks as needed based on clinical and laboratory response.
Children at risk for hyperactivity: To minimize the risk of hyperactivity in children, start at one-fourth the recommended full replacement dose, and increase on a weekly basis by one-fourth the full recommended replacement dose until the full recommended replacement dose is reached.
Pregnancy
Pre-existing Hypothyroidism: Levothyroxine sodium tablets dose requirements may increase during pregnancy. Measure serum TSH and free-T4 as soon as pregnancy is confirmed and, at minimum, during each trimester of pregnancy. In patients with primary hypothyroidism, maintain serum TSH in the trimester-specific reference range. For patients with serum TSH above the normal trimester-specific range, increase the dose of levothyroxine sodium tablets by 12.5 mcg/day to 25 mcg/day and measure TSH every 4 weeks until a stable levothyroxine sodium tablets dose is reached and serum TSH is within the normal trimester-specific range. Reduce levothyroxine sodium tablets dosage to pre-pregnancy levels immediately after delivery and measure serum TSH levels 4 to 8 weeks postpartum to ensure levothyroxine sodium tablets dose is appropriate.
New Onset Hypothyroidism: Normalize thyroid function as rapidly as possible. In patients with moderate to severe signs and symptoms of hypothyroidism, start levothyroxine sodium tablets at the full replacement dose (1.6 mcg per kg body weight per day). In patients with mild hypothyroidism (TSH less than 10 units per liter) start levothyroxine sodium tablets at 1 mcg per kg body weight per day. Evaluate serum TSH every 4 weeks and adjust levothyroxine sodium tablets dosage until a serum TSH is within the normal trimester specific range [see Use in Specific Populations (8.1)].
TSH Suppression in Well-differentiated Thyroid Cancer
Generally, TSH is suppressed to below 0.1 units per liter, and this usually requires a levothyroxine sodium tablets dose of greater than 2 mcg per kg per day. However, in patients with high-risk tumors, the target level for TSH suppression may be lower.
Close2.4 Monitoring TSH and/or Thyroxine (T4) Levels
Assess the adequacy of therapy by periodic assessment of laboratory tests and clinical evaluation. Persistent clinical and laboratory evidence of hypothyroidism despite an apparent adequate replacement dose of levothyroxine sodium tablets may be evidence of inadequate absorption, poor compliance, drug interactions, or a combination of these factors.
Adults
In adult patients with primary hypothyroidism, monitor serum TSH levels after an interval of 6 to 8 weeks after any change in dose. In patients on a stable and appropriate replacement dose, evaluate clinical and biochemical response every 6 to 12 months and whenever there is a change in the patient’s clinical status.
Pediatrics
In patients with congenital hypothyroidism, assess the adequacy of replacement therapy by measuring both serum TSH and total or free-T4. Monitor TSH and total or free-T4 in children as follows: 2 and 4 weeks after the initiation of treatment, 2 weeks after any change in dosage, and then every 3 to 12 months thereafter following dose stabilization until growth is completed. Poor compliance or abnormal values may necessitate more frequent monitoring. Perform routine clinical examination, including assessment of development, mental and physical growth, and bone maturation, at regular intervals.
While the general aim of therapy is to normalize the serum TSH level, TSH may not normalize in some patients due to in utero hypothyroidism causing a resetting of pituitary-thyroid feedback. Failure of the serum T4 to increase into the upper half of the normal range within 2 weeks of initiation of levothyroxine sodium tablets therapy and/or of the serum TSH to decrease below 20 units per liter within 4 weeks may indicate the child is not receiving adequate therapy. Assess compliance, dose of medication administered, and method of administration prior to increasing the dose of levothyroxine sodium tablets [see Warnings and Precautions (5.4) and Use in Specific Populations (8.4)].
Secondary and Tertiary Hypothyroidism
Monitor serum free-T4 levels and maintain in the upper half of the normal range in these patients.
-
3 DOSAGE FORMS AND STRENGTHSLevothyroxine sodium tablets, USP are available containing 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg or 300 mcg of levothyroxine sodium ...
Levothyroxine sodium tablets, USP are available containing 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg or 300 mcg of levothyroxine sodium, USP.
- The 25 mcg tablets are orange color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 1” on the other side.
- The 50 mcg tablets are white color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 2” on the other side.
- The 75 mcg tablets are violet color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 3” on the other side.
- The 88 mcg tablets are olive color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 4” on the other side.
- The 100 mcg tablets are yellow color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 5” on the other side.
- The 112 mcg tablets are rose color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 6” on the other side.
- The 125 mcg tablets are gray color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 7” on the other side.
- The 137 mcg tablets are turquoise color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 8” on the other side.
- The 150 mcg tablets are blue color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 9” on the other side.
- The 175 mcg tablets are lilac color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 0” on the other side.
- The 200 mcg tablets are pink color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 1” on the other side.
- The 300 mcg tablets are green color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 2” on the other side.
-
4 CONTRAINDICATIONSLevothyroxine sodium tablets are contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
Levothyroxine sodium tablets are contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
Close -
5 WARNINGS AND PRECAUTIONS5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease - Over-treatment with levothyroxine may cause an increase in heart rate, cardiac wall ...
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
Over-treatment with levothyroxine may cause an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias, particularly in patients with cardiovascular disease and in elderly patients. Initiate levothyroxine sodium tablets therapy in this population at lower doses than those recommended in younger individuals or in patients without cardiac disease [see Dosage and Administration (2.3), Use in Specific Populations (8.5)].
Monitor for cardiac arrhythmias during surgical procedures in patients with coronary artery disease receiving suppressive levothyroxine sodium tablets therapy. Monitor patients receiving concomitant levothyroxine sodium tablets and sympathomimetic agents for signs and symptoms of coronary insufficiency.
If cardiac symptoms develop or worsen, reduce the levothyroxine sodium tablets dose or withhold for one week and restart at a lower dose.
5.2 Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism, and may result in unpredictable absorption of levothyroxine sodium from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with levothyroxine sodium tablets [see Contraindications (4)].
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
Levothyroxine sodium tablets have a narrow therapeutic index. Over-or undertreatment with levothyroxine sodium tablets may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, emotional state, gastrointestinal function, and glucose and lipid metabolism. Titrate the dose of levothyroxine sodium tablets carefully and monitor response to titration to avoid these effects [see Dosage and Administration (2.4)]. Monitor for the presence of drug or food interactions when using levothyroxine sodium tablets and adjust the dose as necessary [see Drug Interactions (7.9) and Clinical Pharmacology (12.3)].
5.5 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing levothyroxine sodium tablets [see Drug Interactions (7.2)].
Close5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-Replacement
Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in post-menopausal women. The increased bone resorption may be associated with increased serum levels and urinary excretion of calcium and phosphorous, elevations in bone alkaline phosphatase, and suppressed serum parathyroid hormone levels. Administer the minimum dose of levothyroxine sodium tablets that achieves the desired clinical and biochemical response to mitigate this risk.
-
6 ADVERSE REACTIONSAdverse reactions associated with levothyroxine sodium tablets therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5), Overdosage (10)] ...
Adverse reactions associated with levothyroxine sodium tablets therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5), Overdosage (10)]. They include the following:
- General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
- Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
- Musculoskeletal: tremors, muscle weakness, muscle spasm
- Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
- Respiratory: dyspnea
- Gastrointestinal: diarrhea, vomiting, abdominal cramps, elevations in liver function tests
- Dermatologic: hair loss, flushing, rash
- Endocrine: decreased bone mineral density
- Reproductive: menstrual irregularities, impaired fertility
Seizures have been reported rarely with the institution of levothyroxine therapy.
Adverse Reactions in Children
Pseudotumor cerebri and slipped capital femoral epiphysis have been reported in children receiving levothyroxine therapy. Overtreatment may result in craniosynostosis in infants and premature closure of the epiphyses in children with resultant compromised adult height.
Hypersensitivity Reactions
Hypersensitivity reactions to inactive ingredients have occurred in patients treated with thyroid hormone products. These include urticaria, pruritus, skin rash, flushing, angioedema, various gastrointestinal symptoms (abdominal pain, nausea, vomiting and diarrhea), fever, arthralgia, serum sickness, and wheezing. Hypersensitivity to levothyroxine itself is not known to occur.
Close -
7 DRUG INTERACTIONS7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics - Many drugs can exert effects on thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism ...
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
Many drugs can exert effects on thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to levothyroxine sodium tablets (see Tables 2-5 below).
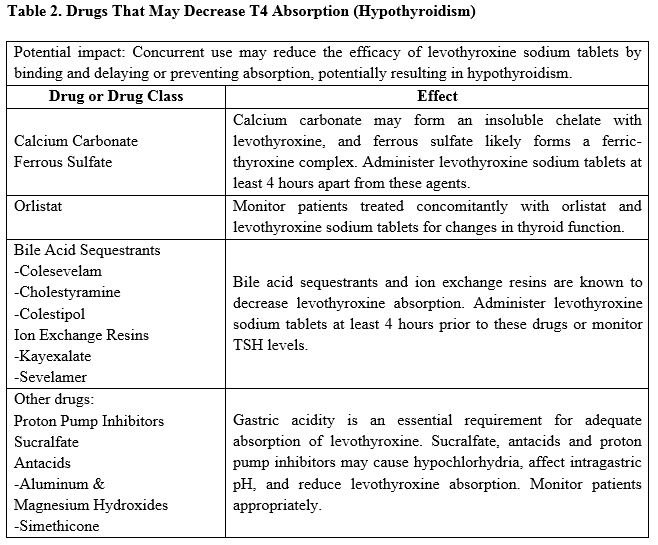
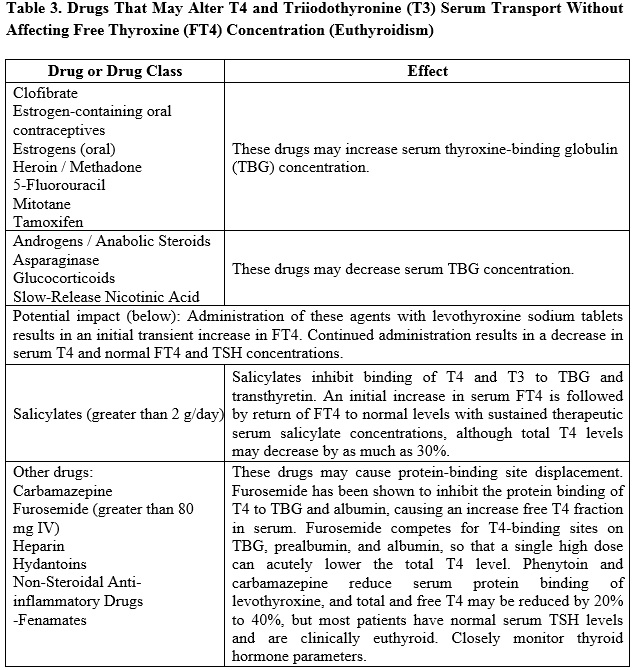
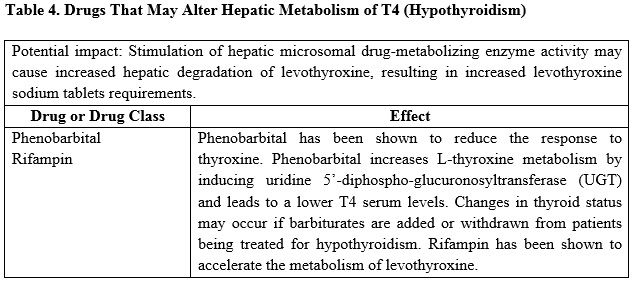

7.2 Antidiabetic Therapy
Addition of levothyroxine sodium tablets therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control, especially when thyroid therapy is started, changed, or discontinued [see Warnings and Precautions (5.5)].
7.3 Oral Anticoagulants
Levothyroxine sodium tablets increase the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the levothyroxine sodium tablets dose is increased. Closely monitor coagulation tests to permit appropriate and timely dosage adjustments.
7.4 Digitalis Glycosides
Levothyroxine sodium tablets may reduce the therapeutic effects of digitalis glycosides. Serum digitalis glycoside levels may decrease when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
7.5 Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and levothyroxine sodium tablets may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and central nervous system stimulation. Levothyroxine sodium tablets may accelerate the onset of action of tricyclics. Administration of sertraline in patients stabilized on levothyroxine sodium tablets may result in increased levothyroxine sodium tablets.
7.6 Ketamine
Concurrent use of ketamine and levothyroxine sodium tablets may produce marked hypertension and tachycardia. Closely monitor blood pressure and heart rate in these patients.
7.7 Sympathomimetics
Concurrent use of sympathomimetics and levothyroxine sodium tablets may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
7.8 Tyrosine-Kinase Inhibitors
Concurrent use of tyrosine-kinase inhibitors such as imatinib may cause hypothyroidism. Closely monitor TSH levels in such patients.
7.9 Drug-Food Interactions
Consumption of certain foods may affect levothyroxine sodium tablets absorption thereby necessitating adjustments in dosing [see Dosage and Administration (2.1)]. Soybean flour, cottonseed meal, walnuts, and dietary fiber may bind and decrease the absorption of levothyroxine sodium tablets from the gastrointestinal tract. Grapefruit juice may delay the absorption of levothyroxine and reduce its bioavailability.
Close7.10 Drug-Laboratory Test Interactions
Consider changes in TBG concentration when interpreting T4 and T3 values. Measure and evaluate unbound (free) hormone and/or determine the free-T4 index (FT4I) in this circumstance. Pregnancy, infectious hepatitis, estrogens, estrogen-containing oral contraceptives, and acute intermittent porphyria increase TBG concentration. Nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, androgens, and corticosteroids decrease TBG concentration. Familial hyper-or hypo-thyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Experience with levothyroxine use in pregnant women, including data from post-marketing studies, have not reported increased rates of major birth defects or ...
8.1 Pregnancy
Risk Summary
Experience with levothyroxine use in pregnant women, including data from post-marketing studies, have not reported increased rates of major birth defects or miscarriages [see Data]. There are risks to the mother and fetus associated with untreated hypothyroidism in pregnancy. Since TSH levels may increase during pregnancy, TSH should be monitored and levothyroxine sodium tablets dosage adjusted during pregnancy [see Clinical Considerations]. There are no animal studies conducted with levothyroxine during pregnancy. Levothyroxine sodium tablets should not be discontinued during pregnancy and hypothyroidism diagnosed during pregnancy should be promptly treated.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Maternal hypothyroidism during pregnancy is associated with a higher rate of complications, including spontaneous abortion, gestational hypertension, pre-eclampsia, stillbirth, and premature delivery. Untreated maternal hypothyroidism may have an adverse effect on fetal neurocognitive development.
Dose Adjustments During Pregnancy and the Postpartum Period
Pregnancy may increase levothyroxine sodium tablets requirements. Serum TSH levels should be monitored and the levothyroxine sodium tablets dosage adjusted during pregnancy. Since postpartum TSH levels are similar to preconception values, the levothyroxine sodium tablets dosage should return to the pre-pregnancy dose immediately after delivery [see Dosage and Administration (2.3)].
Data
Human Data
Levothyroxine is approved for use as a replacement therapy for hypothyroidism. There is a long experience of levothyroxine use in pregnant women, including data from post-marketing studies that have not reported increased rates of fetal malformations, miscarriages or other adverse maternal or fetal outcomes associated with levothyroxine use in pregnant women.
8.2 Lactation
Risk Summary
Limited published studies report that levothyroxine is present in human milk. However, there is insufficient information to determine the effects of levothyroxine on the breastfed infant and no available information on the effects of levothyroxine on milk production. Adequate levothyroxine treatment during lactation may normalize milk production in hypothyroid lactating mothers. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levothyroxine sodium tablets and any potential adverse effects on the breastfed infant from levothyroxine sodium tablets or from the underlying maternal condition.
8.4 Pediatric Use
The initial dose of levothyroxine sodium tablets varies with age and body weight. Dosing adjustments are based on an assessment of the individual patient's clinical and laboratory parameters [see Dosage and Administration (2.3, 2.4)].
In children in whom a diagnosis of permanent hypothyroidism has not been established, discontinue levothyroxine sodium tablets administration for a trial period, but only after the child is at least 3 years of age. Obtain serum T4 and TSH levels at the end of the trial period, and use laboratory test results and clinical assessment to guide diagnosis and treatment, if warranted.
Congenital Hypothyroidism [See Dosage and Administration (2.3, 2.4)]
Rapid restoration of normal serum T4 concentrations is essential for preventing the adverse effects of congenital hypothyroidism on intellectual development as well as on overall physical growth and maturation. Therefore, initiate levothyroxine sodium tablets therapy immediately upon diagnosis. Levothyroxine is generally continued for life in these patients.
Closely monitor infants during the first 2 weeks of levothyroxine sodium tablets therapy for cardiac overload, arrhythmias, and aspiration from avid suckling.
Closely monitor patients to avoid undertreatment or overtreatment. Undertreatment may have deleterious effects on intellectual development and linear growth. Overtreatment is associated with craniosynostosis in infants, may adversely affect the tempo of brain maturation, and may accelerate the bone age and result in premature epiphyseal closure and compromised adult stature.
Acquired Hypothyroidism in Pediatric Patients
Closely monitor patients to avoid undertreatment and overtreatment. Undertreatment may result in poor school performance due to impaired concentration and slowed mentation and in reduced adult height. Overtreatment may accelerate the bone age and result in premature epiphyseal closure and compromised adult stature.
Treated children may manifest a period of catch-up growth, which may be adequate in some cases to normalize adult height. In children with severe or prolonged hypothyroidism, catch-up growth may not be adequate to normalize adult height.
Close8.5 Geriatric Use
Because of the increased prevalence of cardiovascular disease among the elderly, initiate levothyroxine sodium tablets at less than the full replacement dose [see Warnings and Precautions (5.1) and Dosage and Administration (2.3)]. Atrial arrhythmias can occur in elderly patients. Atrial fibrillation is the most common of the arrhythmias observed with levothyroxine overtreatment in the elderly.
-
10 OVERDOSAGEThe signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral ...
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures occurred in a 3-year-old child ingesting 3.6 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Reduce the levothyroxine sodium tablets dose or discontinue temporarily if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient’s medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
Close -
11 DESCRIPTIONLevothyroxine sodium tablets, USP contain synthetic crystalline L-3,3',5,5'-tetraiodothyronine sodium salt. Synthetic T4 is identical in chemical structure to the T4 produced in the human thyroid ...
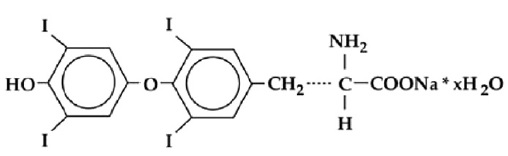
Levothyroxine sodium tablets, USP contain synthetic crystalline L-3,3',5,5'-tetraiodothyronine sodium salt. Synthetic T4 is identical in chemical structure to the T4 produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C15H10I4NNaO4• xH2O, molecular weight of 798.86 g/mol (anhydrous), and structural formula as shown:
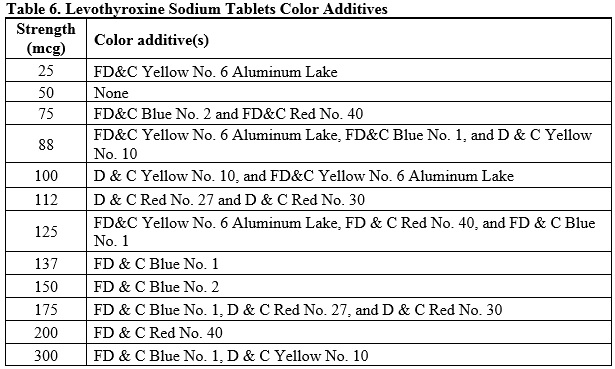
Levothyroxine sodium tablets for oral administration are available in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg, and 300 mcg. Each levothyroxine sodium tablet contains the inactive ingredients butylated hydroxyanisole, microcrystalline cellulose, sodium starch glycolate, povidone, colloidal silicon dioxide, magnesium stearate and color additive(s). Table 6 provides a listing of the color additives by tablet strength:
FDA approved Dissolution test differs from the USP dissolution test.
FDA approved Assay test differs from the USP assay test.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the ...
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Oral levothyroxine sodium is a synthetic T4 hormone that exerts the same physiologic effect as endogenous T4, thereby maintaining normal T4 levels when a deficiency is present.
Close12.3 Pharmacokinetics
Absorption
Absorption of orally administered T4 from the gastrointestinal tract ranges from 40% to 80%. The majority of the levothyroxine sodium tablets dose is absorbed from the jejunum and upper ileum. The relative bioavailability of levothyroxine sodium tablets, compared to an equal nominal dose of oral levothyroxine sodium solution, is approximately 94%. T4 absorption is increased by fasting, and decreased in malabsorption syndromes and by certain foods such as soybeans. Dietary fiber decreases bioavailability of T4. Absorption may also decrease with age. In addition, many drugs and foods affect T4 absorption [see Drug Interactions (7)].
Distribution
Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA), and albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T4 partially explains the higher serum levels, slower metabolic clearance, and longer half-life of T4 compared to T3. Protein-bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Drug Interactions (7)]. Thyroid hormones do not readily cross the placental barrier [see Use in Specific Populations (8.1)].
Elimination
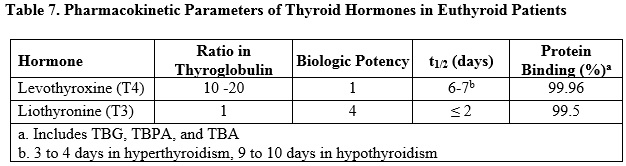
Metabolism
T4 is slowly eliminated (see Table 7). The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately 80% of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (rT3). T3 and rT3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Excretion
Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged and is eliminated in the feces. Approximately 20% of T4 is eliminated in the stool. Urinary excretion of T4 decreases with age.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Standard animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of levothyroxine.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLevothyroxine sodium tablets, USP are available containing 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg or 300 mcg of levothyroxine sodium, USP ...
Levothyroxine sodium tablets, USP are available containing 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg or 300 mcg of levothyroxine sodium, USP. They are available as follows:
The 25 mcg tablets are orange color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 1” on the other side.
NDC 31722-284-30 bottles of 30 tablets
NDC 31722-284-90 bottles of 90 tablets
NDC 31722-284-01 bottles of 100 tablets
NDC 31722-284-10 bottles of 1000 tabletsThe 50 mcg tablets are white color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 2” on the other side.
NDC 31722-285-30 bottles of 30 tablets
NDC 31722-285-90 bottles of 90 tablets
NDC 31722-285-01 bottles of 100 tablets
NDC 31722-285-10 bottles of 1000 tabletsThe 75 mcg tablets are violet color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 3” on the other side.
NDC 31722-286-30 bottles of 30 tablets
NDC 31722-286-90 bottles of 90 tablets
NDC 31722-286-01 bottles of 100 tablets
NDC 31722-286-10 bottles of 1000 tabletsThe 88 mcg tablets are olive color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 4” on the other side.
NDC 31722-287-30 bottles of 30 tablets
NDC 31722-287-90 bottles of 90 tablets
NDC 31722-287-01 bottles of 100 tablets
NDC 31722-287-10 bottles of 1000 tabletsThe 100 mcg tablets are yellow color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 5” on the other side.
NDC 31722-288-30 bottles of 30 tablets
NDC 31722-288-90 bottles of 90 tablets
NDC 31722-288-01 bottles of 100 tablets
NDC 31722-288-10 bottles of 1000 tabletsThe 112 mcg tablets are rose color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 6” on the other side.
NDC 31722-289-30 bottles of 30 tablets
NDC 31722-289-90 bottles of 90 tablets
NDC 31722-289-01 bottles of 100 tablets
NDC 31722-289-10 bottles of 1000 tabletsThe 125 mcg tablets are gray color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 7” on the other side.
NDC 31722-290-30 bottles of 30 tablets
NDC 31722-290-90 bottles of 90 tablets
NDC 31722-290-01 bottles of 100 tablets
NDC 31722-290-10 bottles of 1000 tabletsThe 137 mcg tablets are turquoise color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 8” on the other side.
NDC 31722-291-30 bottles of 30 tablets
NDC 31722-291-90 bottles of 90 tablets
NDC 31722-291-01 bottles of 100 tablets
NDC 31722-291-10 bottles of 1000 tabletsThe 150 mcg tablets are blue color, capsule shaped, biconvex tablets, plain on one side and debossed “score line 9” on the other side.
NDC 31722-292-30 bottles of 30 tablets
NDC 31722-292-90 bottles of 90 tablets
NDC 31722-292-01 bottles of 100 tablets
NDC 31722-292-10 bottles of 1000 tabletsThe 175 mcg tablets are lilac color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 0” on the other side.
NDC 31722-293-30 bottles of 30 tablets
NDC 31722-293-90 bottles of 90 tablets
NDC 31722-293-01 bottles of 100 tablets
NDC 31722-293-10 bottles of 1000 tabletsThe 200 mcg tablets are pink color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 1” on the other side.
NDC 31722-294-30 bottles of 30 tablets
NDC 31722-294-90 bottles of 90 tablets
NDC 31722-294-01 bottles of 100 tablets
NDC 31722-294-10 bottles of 1000 tabletsThe 300 mcg tablets are green color, capsule shaped, biconvex tablets, plain on one side and debossed “1 score line 2” on the other side.
NDC 31722-295-30 bottles of 30 tablets
NDC 31722-295-90 bottles of 90 tablets
NDC 31722-295-01 bottles of 100 tablets
NDC 31722-295-10 bottles of 1000 tabletsStorage Conditions
Store at USP controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C and 30°C (59°F to 86°F) [see USP controlled room temperature]. Levothyroxine sodium tablets should be protected from light and moisture.
Close -
17 PATIENT COUNSELING INFORMATIONInform the patient of the following information to aid in the safe and effective use of levothyroxine sodium tablets: Dosing and Administration - Instruct patients to take levothyroxine ...
Inform the patient of the following information to aid in the safe and effective use of levothyroxine sodium tablets:
Dosing and Administration
- Instruct patients to take levothyroxine sodium tablets only as directed by their healthcare provider.
- Instruct patients to take levothyroxine sodium tablets as a single dose, preferably on an empty stomach, one-half to one hour before breakfast.
- Inform patients that agents such as iron and calcium supplements and antacids can decrease the absorption of levothyroxine. Instruct patients not to take levothyroxine sodium tablets within 4 hours of these agents.
- Instruct patients to notify their healthcare provider if they are pregnant or breastfeeding or are thinking of becoming pregnant while taking levothyroxine sodium tablets.
Important Information
- Inform patients that it may take several weeks before they notice an improvement in symptoms.
- Inform patients that the levothyroxine in levothyroxine sodium tablets is intended to replace a hormone that is normally produced by the thyroid gland. Generally, replacement therapy is to be taken for life.
- Inform patients that levothyroxine sodium tablets should not be used as a primary or adjunctive therapy in a weight control program.
- Instruct patients to notify their healthcare provider if they are taking any other medications, including prescription and over-the-counter preparations.
- Instruct patients to notify their physician of any other medical conditions they may have, particularly heart disease, diabetes, clotting disorders, and adrenal or pituitary gland problems, as the dose of medications used to control these other conditions may need to be adjusted while they are taking levothyroxine sodium tablets. If they have diabetes, instruct patients to monitor their blood and/or urinary glucose levels as directed by their physician and immediately report any changes to their physician. If patients are taking anticoagulants, their clotting status should be checked frequently.
- Instruct patients to notify their physician or dentist that they are taking levothyroxine sodium tablets prior to any surgery.
Adverse Reactions
- Instruct patients to notify their healthcare provider if they experience any of the following symptoms: rapid or irregular heartbeat, chest pain, shortness of breath, leg cramps, headache, nervousness, irritability, sleeplessness, tremors, change in appetite, weight gain or loss, vomiting, diarrhea, excessive sweating, heat intolerance, fever, changes in menstrual periods, hives or skin rash, or any other unusual medical event.
- Inform patients that partial hair loss may occur rarely during the first few months of levothyroxine sodium tablets therapy, but this is usually temporary.
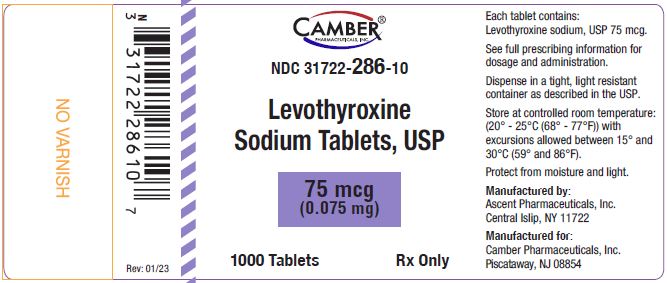
Manufactured by:
Ascent Pharmaceuticals, Inc.
Central Islip, NY 11722Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854Revision: 01/23
Close -
PRINCIPAL DISPLAY PANELLevothyroxine Sodium Tablets, USP - 25 mcg - 90 count - Levothyroxine Sodium Tablets, USP - 25 mcg - 1000 count - Levothyroxine Sodium Tablets, USP - 50 mcg - 90 count - Levothyroxine ...
Levothyroxine Sodium Tablets, USP
25 mcg - 90 count
Levothyroxine Sodium Tablets, USP
25 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
50 mcg - 90 count
Levothyroxine Sodium Tablets, USP
50 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
75 mcg - 90 count
Levothyroxine Sodium Tablets, USP
75 mcg - 1000 count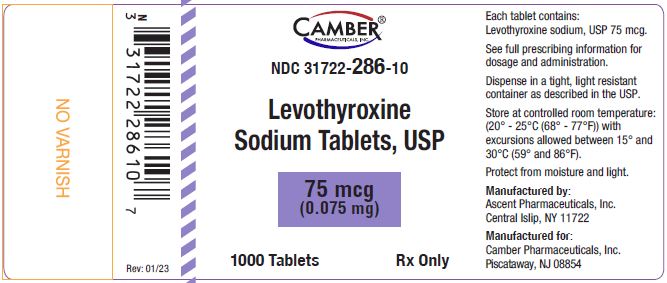
Levothyroxine Sodium Tablets, USP
88 mcg - 90 count
Levothyroxine Sodium Tablets, USP
88 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
100 mcg - 90 count
Levothyroxine Sodium Tablets, USP
100 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
112 mcg - 90 count
Levothyroxine Sodium Tablets, USP
112 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
125 mcg - 90 count
Levothyroxine Sodium Tablets, USP
112 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
137 mcg - 90 count
Levothyroxine Sodium Tablets, USP
137 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
150 mcg - 90 count
Levothyroxine Sodium Tablets, USP
150 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
175 mcg - 90 count
Levothyroxine Sodium Tablets, USP
175 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
200 mcg - 90 count
Levothyroxine Sodium Tablets, USP
200 mcg - 1000 count
Levothyroxine Sodium Tablets, USP
300 mcg - 90 count
Levothyroxine Sodium Tablets, USP
300 mcg - 1000 count
Close
-
INGREDIENTS AND APPEARANCEProduct Information
LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-284 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.025 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-284-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-284-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-284-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-284-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-285 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.05 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-285-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-285-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-285-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-285-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-286 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.075 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color purple (violet) Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-286-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-286-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-286-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-286-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-287 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.088 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color green (olive) Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-287-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-287-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-287-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-287-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-288 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.1 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color yellow Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-288-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-288-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-288-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-288-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-289 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.112 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 30 (UNII: 2S42T2808B) Product Characteristics Color pink (rose) Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-289-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-289-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-289-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-289-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-290 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.125 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color gray Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-290-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-290-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-290-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-290-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-291 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.137 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color turquoise Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-291-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-291-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-291-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-291-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-292 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.15 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) povidone (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-292-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-292-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-292-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-292-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-293 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.175 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 30 (UNII: 2S42T2808B) Product Characteristics Color purple (lilac) Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 1;0 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-293-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-293-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-293-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-293-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-294 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.2 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color pink Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 1;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-294-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-294-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-294-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-294-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 LEVOTHYROXINE SODIUM levothyroxine sodium tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-295 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 0.3 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color green Score 2 pieces Shape capsule Size 9mm Flavor Imprint Code 1;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-295-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 2 NDC:31722-295-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 3 NDC:31722-295-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 4 NDC:31722-295-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215259 01/18/2023 Labeler - Camber Pharmaceuticals, Inc. (826774775)
CloseEstablishment Name Address ID/FEI Business Operations Ascent Pharmaceuticals, Inc 080938961 manufacture(31722-284, 31722-285, 31722-286, 31722-287, 31722-288, 31722-289, 31722-290, 31722-291, 31722-292, 31722-293, 31722-294, 31722-295) , analysis(31722-284, 31722-285, 31722-286, 31722-287, 31722-288, 31722-289, 31722-290, 31722-291, 31722-292, 31722-293, 31722-294, 31722-295) , pack(31722-284, 31722-285, 31722-286, 31722-287, 31722-288, 31722-289, 31722-290, 31722-291, 31722-292, 31722-293, 31722-294, 31722-295)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LEVOTHYROXINE SODIUM tablet
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jan 24, 2023 | 1 (current) | download |
RxNorm
LEVOTHYROXINE SODIUM tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 892246 | levothyroxine sodium 100 MCG Oral Tablet | PSN |
| 2 | 892246 | levothyroxine sodium 0.1 MG Oral Tablet | SCD |
| 3 | 892246 | levothyroxine sodium 100 MCG Oral Tablet | SY |
| 4 | 892251 | levothyroxine sodium 200 MCG Oral Tablet | PSN |
| 5 | 892251 | levothyroxine sodium 0.2 MG Oral Tablet | SCD |
| 6 | 892251 | levothyroxine sodium 200 MCG Oral Tablet | SY |
| 7 | 892255 | levothyroxine sodium 300 MCG Oral Tablet | PSN |
| 8 | 892255 | levothyroxine sodium 0.3 MG Oral Tablet | SCD |
| 9 | 892255 | levothyroxine sodium 300 MCG Oral Tablet | SY |
| 10 | 966220 | levothyroxine sodium 25 MCG Oral Tablet | PSN |
| 11 | 966220 | levothyroxine sodium 0.025 MG Oral Tablet | SCD |
| 12 | 966220 | levothyroxine sodium 25 MCG Oral Tablet | SY |
| 13 | 966221 | levothyroxine sodium 50 MCG Oral Tablet | PSN |
| 14 | 966221 | levothyroxine sodium 0.05 MG Oral Tablet | SCD |
| 15 | 966221 | levothyroxine sodium 50 MCG Oral Tablet | SY |
| 16 | 966222 | levothyroxine sodium 75 MCG Oral Tablet | PSN |
| 17 | 966222 | levothyroxine sodium 0.075 MG Oral Tablet | SCD |
| 18 | 966222 | levothyroxine sodium 75 MCG Oral Tablet | SY |
| 19 | 966224 | levothyroxine sodium 125 MCG Oral Tablet | PSN |
| 20 | 966224 | levothyroxine sodium 0.125 MG Oral Tablet | SCD |
| 21 | 966224 | levothyroxine sodium 125 MCG Oral Tablet | SY |
| 22 | 966225 | levothyroxine sodium 150 MCG Oral Tablet | PSN |
| 23 | 966225 | levothyroxine sodium 0.15 MG Oral Tablet | SCD |
| 24 | 966225 | levothyroxine sodium 150 MCG Oral Tablet | SY |
| 25 | 966248 | levothyroxine sodium 112 MCG Oral Tablet | PSN |
| 26 | 966248 | levothyroxine sodium 0.112 MG Oral Tablet | SCD |
| 27 | 966248 | levothyroxine sodium 112 MCG Oral Tablet | SY |
| 28 | 966249 | levothyroxine sodium 175 MCG Oral Tablet | PSN |
| 29 | 966249 | levothyroxine sodium 0.175 MG Oral Tablet | SCD |
| 30 | 966249 | levothyroxine sodium 175 MCG Oral Tablet | SY |
| 31 | 966253 | levothyroxine sodium 88 MCG Oral Tablet | PSN |
| 32 | 966253 | levothyroxine sodium 0.088 MG Oral Tablet | SCD |
| 33 | 966253 | levothyroxine sodium 88 MCG Oral Tablet | SY |
| 34 | 966270 | levothyroxine sodium 137 MCG Oral Tablet | PSN |
| 35 | 966270 | levothyroxine sodium 0.137 MG Oral Tablet | SCD |
| 36 | 966270 | levothyroxine sodium 137 MCG Oral Tablet | SY |
Get Label RSS Feed for this Drug
LEVOTHYROXINE SODIUM tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=b1ba1c7d-371a-4aa8-bee8-dd29b5e7da7e
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LEVOTHYROXINE SODIUM tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 31722-284-01 |
| 2 | 31722-284-10 |
| 3 | 31722-284-30 |
| 4 | 31722-284-90 |
| 5 | 31722-285-01 |
| 6 | 31722-285-10 |
| 7 | 31722-285-30 |
| 8 | 31722-285-90 |
| 9 | 31722-286-01 |
| 10 | 31722-286-10 |
| 11 | 31722-286-30 |
| 12 | 31722-286-90 |
| 13 | 31722-287-01 |
| 14 | 31722-287-10 |
| 15 | 31722-287-30 |
| 16 | 31722-287-90 |
| 17 | 31722-288-01 |
| 18 | 31722-288-10 |
| 19 | 31722-288-30 |
| 20 | 31722-288-90 |
| 21 | 31722-289-01 |
| 22 | 31722-289-10 |
| 23 | 31722-289-30 |
| 24 | 31722-289-90 |
| 25 | 31722-290-01 |
| 26 | 31722-290-10 |
| 27 | 31722-290-30 |
| 28 | 31722-290-90 |
| 29 | 31722-291-01 |
| 30 | 31722-291-10 |
| 31 | 31722-291-30 |
| 32 | 31722-291-90 |
| 33 | 31722-292-01 |
| 34 | 31722-292-10 |
| 35 | 31722-292-30 |
| 36 | 31722-292-90 |
| 37 | 31722-293-01 |
| 38 | 31722-293-10 |
| 39 | 31722-293-30 |
| 40 | 31722-293-90 |
| 41 | 31722-294-01 |
| 42 | 31722-294-10 |
| 43 | 31722-294-30 |
| 44 | 31722-294-90 |
| 45 | 31722-295-01 |
| 46 | 31722-295-10 |
| 47 | 31722-295-30 |
| 48 | 31722-295-90 |