Label: ALLOPURINOL tablet
- NDC Code(s): 31722-252-01, 31722-252-05, 31722-252-10, 31722-252-90, view more
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALLOPURINOL TABLETS safely and effectively. See full prescribing information for ALLOPURINOL TABLETS. ALLOPURINOL tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAllopurinol tablets are indicated for: • The management of adults with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Testing Prior to Treatment Initiation - Prior to initiating treatment with allopurinol tablets in patients with gout, assess the following baseline tests: serum uric acid ...
-
3 DOSAGE FORMS AND STRENGTHSAllopurinol tablets, USP have functional scoring and are available in the following strengths: • 100 mg: White to off-white colored, round tablets debossed with “U” and “5” on one side and ...
-
4 CONTRAINDICATIONSAllopurinol tablets are contraindicated in patients with a history of hypersensitivity reaction to allopurinol or to any of the ingredients of allopurinol tablets.
-
5 WARNINGS AND PRECAUTIONS5.1 Skin Rash and Hypersensitivity - Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and drug reaction with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Skin Rash and Hypersensitivity [see Warnings and Precautions (5.1)] • Nephrotoxicity ...
-
7 DRUG INTERACTIONS7.1 Drugs Known to Affect the Occurrence of Skin Rash and Hypersensitivity - Concomitant use of the following drugs may increase the risk of skin rash, which may be severe: bendamustine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals, allopurinol may cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have been described in ...
-
10 OVERDOSAGEIn the management of overdosage there is no specific antidote for allopurinol. Both allopurinol and oxipurinol are dialyzable; however, the usefulness of hemodialysis or peritoneal dialysis in ...
-
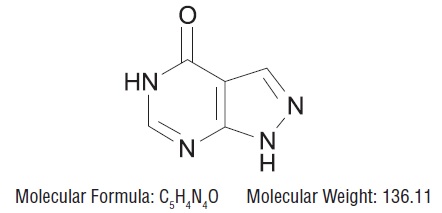
11 DESCRIPTIONAllopurinol, USP is a xanthine oxidase inhibitor. It has the following structural formula: Molecular Formula: C5H4N4O Allopurinol is known chemically as 1, 5-dihydro-4H-pyrazolo [3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Allopurinol is a structural analogue of the natural purine base, hypoxanthine. Allopurinol acts on purine catabolism, without disrupting the biosynthesis of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of tumorigenicity was observed in male or female mice or rats that received oral allopurinol for the majority of their ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Allopurinol Tablets, USP 100 mg -White to off-white colored, round tablets debossed with “U” and “5” on one side and functional score line with “H” on the other side. Bottles of ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Advise patients to take allopurinol tablets after meals to minimize gastric irritation. If a single dose of allopurinol tablets is occasionally forgotten, there is no need to ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELAllopurinol Tablets 100 mg 90s - Allopurinol Tablets 300 mg 90s
-
INGREDIENTS AND APPEARANCEProduct Information