Label: TERBINAFINE HYDROCHLORIDE- terbinafine tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 31722-209-01, 31722-209-05, 31722-209-30 - Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 15, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONTerbinafine tablets, USP 250 mg for oral use - These highlights do not include all the information needed to use Terbinafine Hydrochloride safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Terbinafine tablets, USP are indicated for the treatment of onychomycosis of the toenail or fingernail due to dermatophytes (tinea unguium). Prior to initiating treatment, appropriate nail ...
-
2 DOSAGE AND ADMINISTRATION
Fingernail onychomycosis: One 250 mg tablet once daily for 6 weeks. Toenail onychomycosis: One 250 mg tablet once daily for 12 weeks. The optimal clinical effect is seen some months after ...
-
3 DOSAGE FORMS AND STRENGTHS
Terbinafine tablets, 250 mg are supplied as white, round, flat faced beveled edge tablets debossed with IG on one side and 209 on the other.
-
4 CONTRAINDICATIONS
Terbinafine tablets are contraindicated in individuals with a history of allergic reaction to oral terbinafine because of the risk of anaphylaxis.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity - Cases of liver failure, some leading to liver transplant or death, have occurred with the use of terbinafine tablets in individuals with and without pre-existing liver ...
-
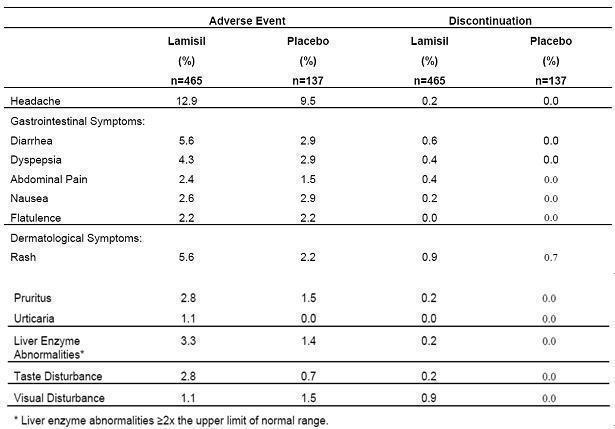
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions - In vivo studies have shown that terbinafine is an inhibitor of the CYP450 2D6 isozyme. Drugs predominantly metabolized by the CYP450 2D6 isozyme include the ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Category B: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response ...
-
10 OVERDOSAGE
Clinical experience regarding overdose with oral terbinafine is limited. Doses up to 5 grams (20 times the therapeutic daily dose) have been taken without inducing serious adverse reactions. The ...
-
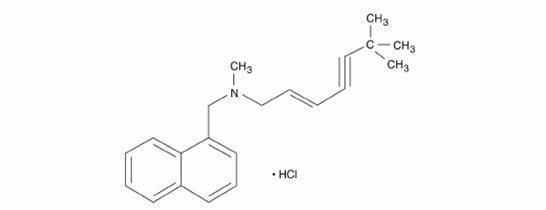
11 DESCRIPTION
Terbinafine tablets contain the synthetic allylamine antifungal compound terbinafine hydrochloride. Chemically, terbinafine hydrochloride, USP is (E)-N-(6 ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Terbinafine is an allylamine antifungal [see Clinical Pharmacology (12.4)]. 12.2 Pharmacodynamics - The pharmacodynamics of terbinafine is unknown ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 28-month oral carcinogenicity study in rats, an increase in the incidence of liver tumors was observed in males at the ...
-
14 CLINICAL STUDIES
The efficacy of terbinafine tablets in the treatment of onychomycosis is illustrated by the response of patients with toenail and/or fingernail infections who participated in three US/Canadian ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Terbinafine tablets, USP are supplied as white, round, flat faced beveled edge tablets debossed with IG on one side and 209 on the other. Bottles of 30 tablets NDC ...
-
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling (Patient Information)] Patients taking terbinafine tablets should receive the following information and instructions: Patients should take one 250 mg tablet ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information