Label: EPLERENONE- eplerenone tablet, film coated
- NDC Code(s): 31722-049-30, 31722-049-90, 31722-050-30, 31722-050-90
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPLERENONE TABLETS safely and effectively. See full prescribing information for EPLERENONE TABLETS. EPLERENONE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Heart Failure Post-Myocardial Infarction - Eplerenone tablets are indicated to improve survival of stable patients with symptomatic heart failure with reduced ejection fraction (≤40% ...
-
2 DOSAGE AND ADMINISTRATION2.1 Heart Failure Post-Myocardial Infarction - Initiate treatment at 25 mg once daily and titrate to the recommended dose of 50 mg once daily, preferably within 4 weeks as tolerated by the ...
-
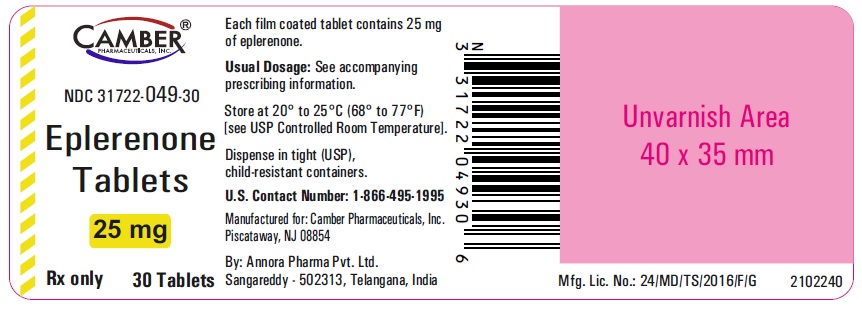
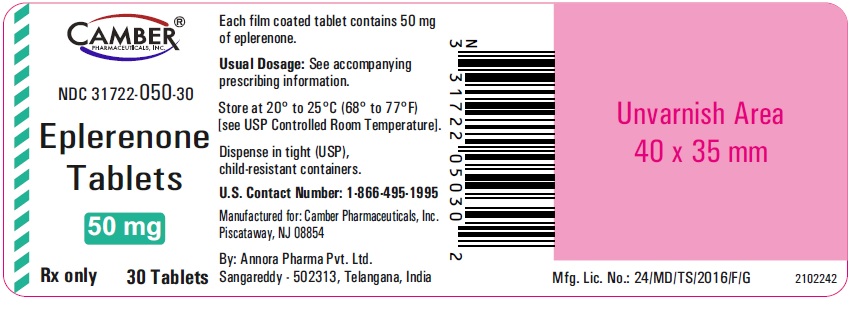
3 DOSAGE FORMS AND STRENGTHS• 25 mg tablets: Light yellow, Round, biconvex, film coated tablets debossed with "V" on one side and :68" on the other side. • 50 mg tablets: Light yellow, Round, biconvex, film coated ...
-
4 CONTRAINDICATIONSFor All Patients - Eplerenone tablets are contraindicated in all patients with: • serum potassium >5.5 mEq/L at initiation, • creatinine clearance ≤30 mL/min, or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyperkalemia - The risk of hyperkalemia is higher in patients with impaired renal function, proteinuria, diabetes and those concomitantly treated with ACEs, ARBs, NSAIDs and moderate CYP3A ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: • Hyperkalemia - [see Warnings and Precautions ( 5.1)] 6.1 Clinical ...
-
7 DRUG INTERACTIONS7.1 CYP3A Inhibitors - Eplerenone metabolism is predominantly mediated via CYP3A. Do not use eplerenone with drugs that are strong inhibitors of CYP3A - [see Contraindications ( 4) and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data from published case reports on eplerenone use during pregnancy are insufficient to establish a drug-associated risk of major birth ...
-
10 OVERDOSAGENo cases of human overdosage with eplerenone have been reported. Lethality was not observed in mice, rats, or dogs after single oral doses that provided C - maxexposures at least 25 times ...
-
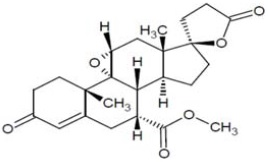
11 DESCRIPTIONEplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone-system (RAAS). Aldosterone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Eplerenone was non-genotoxic in a battery of assays including - in vitrobacterial mutagenesis (Ames test in - Salmonellaspp. and ...
-
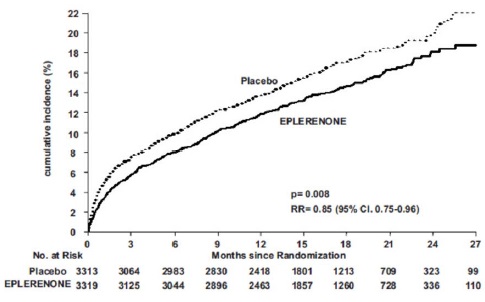
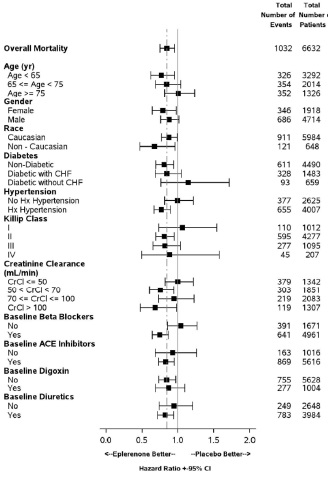
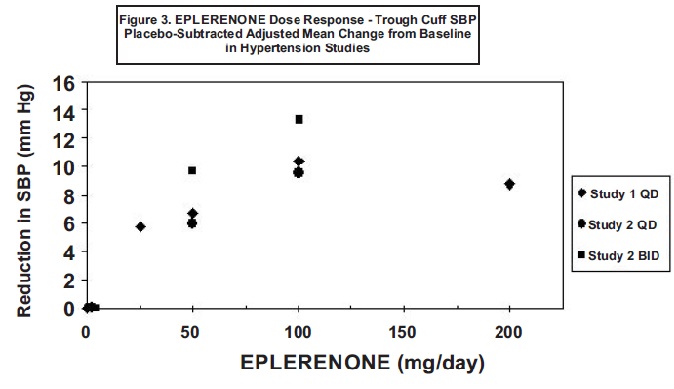
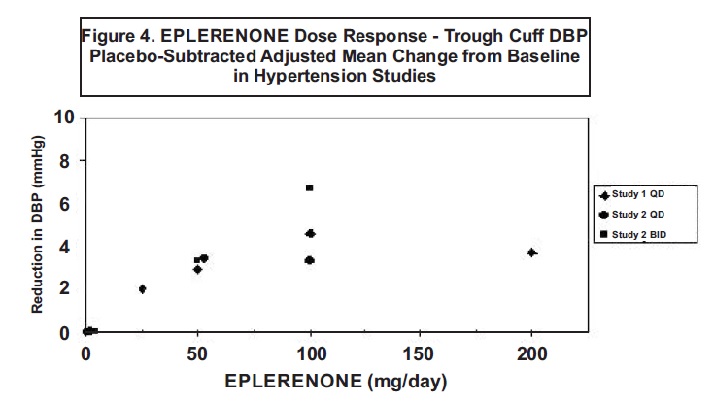
14 CLINICAL STUDIES14.1 Heart Failure Post-Myocardial Infarction - The eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) was a multinational, multicenter ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEplerenone Tablets 25 mgare light yellow, round, biconvex, film coated tablets debossed with "V" on one side and "68" on the other side. Bottle of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients receiving eplerenone: • Not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician - [see Warnings and ...
-
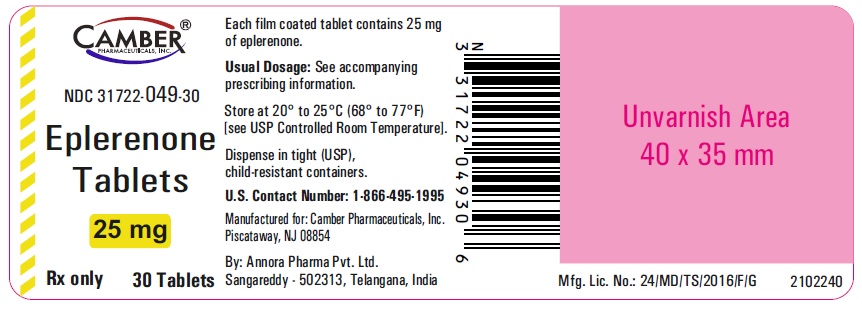
PACKAGE LABEL.PRINCIPAL DISPLAY PANELEplerenone Tablets 25mg 30's Container label - Eplerenone Tablets 50 mg 30's Container label
-
INGREDIENTS AND APPEARANCEProduct Information