Label: CYCLOBENZAPRINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 29300-413-01, 29300-413-05, 29300-413-10, 29300-413-19, view more
- Packager: Unichem Pharmaceuticals (USA), Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
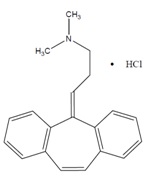
DESCRIPTIONCyclobenzaprine hydrochloride, USP is a white to off-white, odorless, crystalline powder with the molecular formula C20H21N•HCl and a molecular weight of 311.85. It has a melting point between ...
-
CLINICAL PHARMACOLOGYCyclobenzaprine HCl relieves skeletal muscle spasm of local origin without interfering with muscle function. It is ineffective in muscle spasm due to central nervous system ...
-
INDICATIONS AND USAGECyclobenzaprine hydrochloride tablets, USP are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal ...
-
CONTRAINDICATIONSHypersensitivity to any component of this product. Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures, and deaths ...
-
WARNINGSSerotonin Syndrome - The development of a potentially life-threatening serotonin syndrome has been reported with cyclobenzaprine hydrochloride when used in combination with other drugs, such as ...
-
PRECAUTIONSGeneral - Because of its atropine-like action, cyclobenzaprine hydrochloride should be used with caution in patients with a history of urinary retention, angle-closure glaucoma, increased ...
-
ADVERSE REACTIONSIncidence of most common adverse reactions in the 2 double-blind*, placebo-controlled 5 mg studies (incidence of > 3% on cyclobenzaprine hydrochloride 5 mg): *Note: Cyclobenzaprine ...
-
DRUG ABUSE AND DEPENDENCEPharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when cyclobenzaprine hydrochloride is administered, even though they have not been ...
-
OVERDOSAGEAlthough rare, deaths may occur from overdosage with cyclobenzaprine hydrochloride. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. As management of ...
-
DOSAGE AND ADMINISTRATIONFor most patients, the recommended dose of cyclobenzaprine hydrochloride tablets, USP is 5 mg three times a day. Based on individual patient response, the dose may be increased to 10 mg three ...
-
HOW SUPPLIEDCyclobenzaprine hydrochloride tablets, USP are available in 5 mg 7.5 mg and 10 mg dosage strengths. Cyclobenzaprine hydrochloride tablets, USP 5 mg are white to off-white, film coated, round ...
-
SPL UNCLASSIFIED SECTIONSTORAGE - Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Rx Only - Additional patient information leaflets can be obtained by calling Unichem at 1-866 ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information