Label: DOXAZOSIN MESYLATE tablet
- NDC Code(s): 29300-351-01, 29300-351-10, 29300-351-13, 29300-352-01, view more
- Packager: Unichem Pharmaceuticals (USA), Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXAZOSIN TABLETS safely and effectively. See full prescribing information for DOXAZOSIN TABLETS. DOXAZOSIN tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Benign Prostatic Hyperplasia (BPH) Doxazosin tablets are indicated for the treatment of the signs and symptoms of BPH. 1.2 Hypertension - Doxazosin tablets are indicated for the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Following the initial dose and with each dose increase of doxazosin tablets, monitor blood pressure for at least 6 hours followingadministration. If doxazosin tablets ...
-
3 DOSAGE FORMS AND STRENGTHSDoxazosin Tablets, USP are available containing doxazosin mesylate, USP equivalent to 1 mg, 2 mg, 4 mg or 8 mg doxazosin (free base). The 1 mg tablets are orange colored, capsule ...
-
4 CONTRAINDICATIONSThe use of doxazosin tablets are contraindicated in patients with a hypersensitivity to doxazosin, other quinazolines (e.g., prazosin, terazosin), or any of its components.
-
5 WARNINGS AND PRECAUTIONS5.1 Postural Hypotension - Postural hypotension with or without symptoms (e.g., dizziness) may develop within a few hours following administration of doxazosin tablets. However, infrequently ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 CYP 3A Inhibitors - In vitro studies suggest that doxazosin is a substrate of CYP 3A4. Strong CYP3A inhibitors may increase exposure to doxazosin. Monitor blood pressure and for symptoms of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data with doxazosin tablets in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage ...
-
10 OVERDOSAGEExperience with doxazosin tablets overdosage is limited. Two adolescents, who each intentionally ingested 40 mg doxazosin tablets with diclofenac or acetaminophen, were treated with gastric ...
-
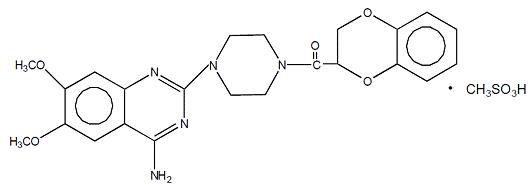
11 DESCRIPTIONDoxazosin tablets, USP are a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha -adrenergic receptors. The chemical name of doxazosin mesylate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Benign Prostatic Hyperplasia (BPH) The symptoms associated with benign prostatic hyperplasia (BPH), such as urinary frequency, nocturia, weak stream, hesitancy ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis: Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses ...
-
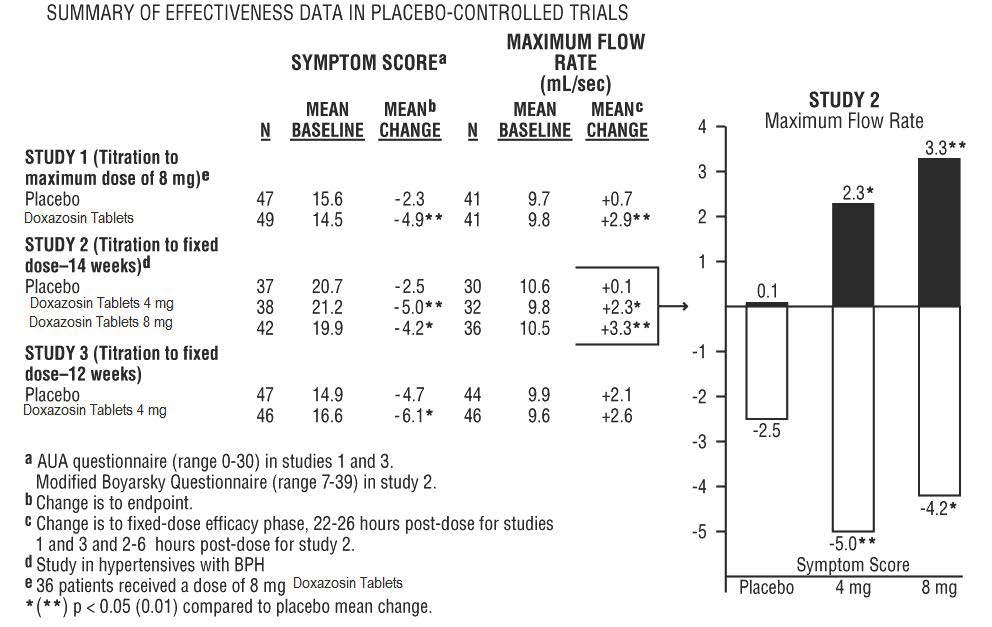
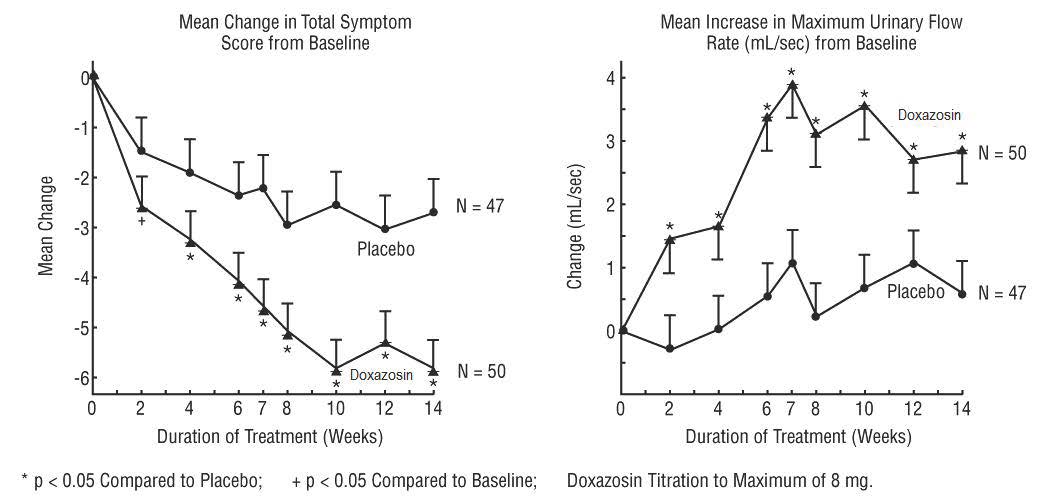
14 CLINICAL STUDIES14.1 Benign Prostatic Hyperplasia (BPH) The efficacy of doxazosin tablets was evaluated extensively in over 900 patients with BPH in double-blind, placebo-controlled trials. Doxazosin tablets ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDoxazosin Tablets, USP are available as scored tablets for oral administration. Each tablet contains doxazosin mesylate equivalent to 1 mg (orange), 2 mg (blue), 4 mg (gray) and 8 mg (white to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Postural Hypotension - Advise patients of the possibility of syncopal and orthostatic symptoms, especially ...
-
PATIENT MEDICATION INFORMATION SECTIONPATIENT INFORMATION - Doxazosin (dox ay' zoe sin) Tablets, USP - What are doxazosin tablets? Doxazosin tablets are a prescription medicine that contains doxazosin mesylate and are ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information